Abstract
Depression is a relatively common agonizing psychiatric disorder that affects the way we feel and think about ourselves and the world around us. Cognitive theories of depression have long posited that various cognitive biases are involved in the development and recurrence of depression. However, the current cognitive theory of depression has been reformulated and expanded from the previous cognitive model of depression based on the results from pharmacological, neuroimaging and neurocognitive studies. This review summarizes the evidence for cognitive dysfunctions in depression and the related pharmacological, neuroanatomical and genetic aspects which aim to integrate our knowledge about the cognitive aspects of depression and its treatment. The newly formulated cognitive theory of depression provides directions for future investigation to identify people at risk, to minimize recurrence, and to maximize long-term beneficial outcomes for those suffering from depression.
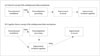
Depression not only changes the way we feel, it also changes how we perceive ourselves and the world around us. Negative views about the self, the world, and the future (Beck’s triad, Fig. 1), as well as uncontrollable recurrent negative thoughts, are agonizing symptoms of depression. Although cognitive disturbance is recognized as an “accompanying” finding of major depressive disorder (MDD) in the current diagnostic criteria [diagnostic and statistical manual of mental disorders, fifth edition (DSM-V)], cognition is regarded as a key component of depression in the cognitive theory of depression.
According to the cognitive theory of depression, people’s attitudes, thoughts, inferences, interpretations, and the way in which they attend to and recall events can trigger depression development and recurrence. Indeed, the cognitive theory embraces vulnerability-stress hypotheses which proposed that the development of depression is due to the interaction of a cognitive vulnerability (e.g., certain cognitions or ways of thought processing information) and a precipitating stressor (e.g., a negative event or some social and environmental factor). Thus, this is a kind of continuum approach which suggests that depression is not qualitatively different from normal mood but quantitatively different from normal mood. According to this theory, one of the most effective interventions for depression is modifying biased cognitive pattern and it claims that reconstructing biased interpretations and dysfunctional automatic thought will result in improvement of other symptoms of the disorder, including sustained negative mood and lack of interest.1
However, the modern cognitive theory of depression has been reconstituted and expanded from Beck’s cognitive model of depression based on the results from recent pharmacological, neuroimaging, neurocognitive and genetic studies. This integrated approach proposes that dysfunction of the monoaminergic neurotransmitter systems leads to alteration in the bottom-up processing of emotional stimuli, which results in negative perceptions during depression. Moreover, the resulting negative biases and schemata themselves also can generate top-down processing manifested as negative expectations which sustain negative schemata.2
In this paper, we integrate more recent studies assessing cognition and depression, and discuss the limitations of work in this field to date. From the previous reports, we review the characteristics of depression that underscore its several key cognitive features. Finally, we briefly discuss the implications of this theory for the treatment of depression and future integrative investigations on the psychological and neuro-biological aspects of this disorder.
MDD is characterized by a set of emotional, behavioral and cognitive symptoms, including psychomotor agitation (or retardation), extreme feelings of guilt (or worthlessness), insomnia (or hypersomnia), fatigue, marked weight loss, decreased appetite, concentration difficulties, and suicidal ideation. Although all these symptoms of MDD are important, depression is basically a disorder of emotional dysregulation and sustained loss of pleasure according to the current diagnostic concept. In DSM-V, application of these core criterion symptoms to the diagnosis of MDD has not changed from that in DSM-IV.
Depression is a highly recurrent disorder. More than 75% of patients with depression have more than one outbreak of depression, often relapsing within two years of remission from depression.3 Such a high recurrence rate suggests that there are specific factors increasing the repeated recurrence of this disorder. Cognitive biases in the processing of emotional information may be the important factors.
In 1976, Beck proposed that existing memory representations (or schemas), lead individuals to filter stimuli from the environment such that their attention is directed toward information that is congruent with their schemas.1 This theory views development and relapse of depression as a result of the persistent, self-reinforcing, maladaptive negative schemata, dysfunctional attitudes and attributional styles. Negative expectations lead to the emergence of depressive thinking processes such as negative emotional biases, negative automatic thoughts or rumination, which consequently contribute to abnormal “hot” cognitive processing in a top-down manner. Accordingly, Beck and other researchers proposed interventions to reconstruct patterns of maladaptive thoughts and behaviors, and they claimed that these changes would improve other symptoms of depression.
The current cognitive theory of depression suggests that the negative schemata are not the direct result of negative early experiences, but instead are triggered by dysfunctional affective processing biases of multiple origins. The most important conceptualized origin of biases may be alterations in monoamine transmission. Although at first glance, this difference may appear negligible, its implications are substantial.
In contrast to the earlier pure psychological theory, the modern cognitive theory of depression embraces recent pharmacological and neurocognitive achievements. This integrated approach postulates that dysfunction of the monoaminergic neurotransmitter systems, which might be related to either environmental or genetic factors or more likely to a combination of both, altered the bottom-up processing of emotional stimuli and this resulted in negative perceptions during depression. Consequent negative biases and schemata resulting from the decreased monoaminergic modulation in neural circuits during emotional processing can be influenced by manipulation of monoaminergic neurotransmission.24
These dysfunctional negative schemata also can generate top-down biases and these manifest as negative expectations which again sustain and enforce negative schemata.2 Selective serotonin reuptake inhibitors can decrease symptoms by influencing bottom-up negative biases; however, this strategy may only be fully successful if correction of their dysfunctional cognitive processes is subsequently reformulaed their top-down biases. This is also supported by the fact that pharmaco-cognitive combination therapy is significantly more effective compared to either method on its own.5 In particular, the cognitive theory approach emphasizes the critical role of negative affective biases in the development and treatment of depression; moreover, it provides a theoretical background in which the traditional purely ‘psychological’ and the recently developed ‘neurochemical’ model of depression might be reconciled.
Although the patients with depression demonstrate diverse cognitive dysfunctions, there are two distinct cognitive dysfunction patterns correlating mood and emotional symptoms of depression and dysphoria. To understand the various subtle cognitive alterations in depression, differentiating affect-independent (or cold) and affect-laden (or hot) cognitive functions is necessary. Cold cognitive dysfunctions in patients with depression are general deficits in cognitive functioning such as difficulties, distractibility, impairments in memory6 and difficulties disengaging from negative images.7 There are hot cognitive dysfunctions in depressed patients; despite these cold cognitive dysfunctions, they exhibit easy concentration on negative self-focused thoughts, and they show enhanced recall of mood-congruent (i.e., negatively valenced) events.8
“Cold” cognition refers to information processing independent of emotional status and can be assessed with commonly used formal neurocognitive tests where the stimulus is emotionally neutral and the result of the test is not generally dependent on motivation.9 On the contrary, “hot” cognition indicates information processing influenced by emotional status and can be recognized during a conversation or in a test related to stimuli carrying emotional valence. In patients with depression, cognitive dysfunctions congruent with mood are reported in several cognitive domains and other domains abnormalities related to cognitive processing of reward and punishment have also been reported. In various perception, memory, attention and working memory tests related to emotional processing, patients with depression give more negatively biased answers. Furthermore, altered performance in reward- and punishment processing was identified in patients with depression, and these findings suggested an enhanced sensitivity towards negative feedback and a reduced sensitivity towards positive feedback, and decreased learning capability related to rewarding cues.269
However, “cold” cognitive deficits observed in patients with depression can also be influenced by alterations in “hot” cognitive processing, i.e., emotion-independent cognitive tasks frequently become emotion-laden in patients with depression, which is especially observed in feedback-based tasks. In response to task failure, catastrophic reaction was frequently observed in patients with depression, i.e., they show a higher error rate in the next trial after one mistake.26 These findings suggest that neurocognitive dysfunctions in patients with depression play a fundamental role in the manifestation of other depressive symptoms.
Certain cognitive deficits in patients with depression may exclusively occur during depressive episodes and are also observed between episodes or even prior to the outbreak of depression. By identifying trait and state cognitive changes, it would be possible to explore these cognitive changes and dysfunctions which are present even preceding the episode of depression. These can also be found in first-degree non-affected relatives and could therefore be considered as trait-like vulnerability markers. Furthermore, it is important to explore and assess these residual cognitive symptoms which are present after the recovery of depressive episodes, since they can profoundly and persistently affect the quality of life and function of patients with depression.
Executive functions are a set of higher-level processes-including attentional control, inhibitory control, working memory, cognitive flexibility, reasoning, problem solving, and planning-that are necessary for the control and coordination with other cognitive abilities and behaviors. These selecting and successful monitoring of behaviors facilitate the attainment of chosen goals.
There is mixed evidence for executive function deficits associated with MDD. Although many studies have reported significant deficits on many neuropsychological tests of executive function, other studies have reported no significant deficit in patients with MDD compared to healthy control participants. However, several recent reviews have found partial support for deficits across multiple domains of executive function, including working memory, shifting, inhibition, planning, and verbal fluency.101112 These deficits were also present in unmedicated MDD patients.13 Moreover, these executive dysfunctions were also present in remitted cases despite improvement of depression,14 particularly in older adults.15
Consistent with these neuropsychological findings, functional imaging and human lesion studies have reported dysfunction of the dorsal and lateral prefrontal cortex in depressed patients performing executive tasks, although these structures interact with subcortical structures and posterior cortical regions.16
Interestingly, the activation of these structures differs according to the cognitive tests. For example, tests of forward planning17 or verbal fluency18 showed attenuated prefrontal activation in the MDD group compared to healthy controls. However, working memory task,19 mental arithmetic task,20 and the Stroop task21 showed greater prefrontal activation in MDD, wherein there were no differences in performance between patients and healthy controls. Although the reason for these inconsistencies may not be clear, exaggerated activation may suggest reduced cortical efficiency; i.e., patients with depression may require greater frontal lobe activation to maintain a comparable level of task performance to that in healthy controls.
Various memory function deficits, including a virtual reality spatial navigation task22 or paragraph recall (remembering the details of a complex story after a 10-min delay), have been reported in patients with depression.23 Moreover, memory impairment is highly predictive of functional outcome and correlates with indices of illness chronicity.24
This pronounced memory deficit may be due to hippocampal dysfunction. Hippocampal function is impaired in patients with MDD during memory encoding tasks,25 and reduced hippocampal volume is arguably the most robust neuropathological finding reported in MDD, supported by meta-analyses of MRI data26 as well as postmortem evidence.27
The symptoms of depression suggest a processing bias toward negative aspects of the environment. For example, more enhanced recall of negative compared to positive material is one of the most consistent findings in depression studies.828 Patients with depression are more likely to recall negative autobiographical memories, and when they recall positive memories, they are lacking in detail, i.e., characteristically overgeneral.29 In contrast, healthy participants typically show a bias for positive material. These findings are more consistently reported in the explicit memory test than in the implicit memory test in patients with depression.
The task of recognizing emotional facial expression or the task that presents emotional words or pictures is a widely used test for affection processing bias in neurocognitive and functional imaging studies. The patients with depression are impaired in recognizing happy facial expressions, whereas manic patients are impaired in recognizing negative (including sad) facial expressions.30 The affective go/no-go test is also used to identify these biases and this test requires the processing of affect in the context of an inhibitory control task. In this test, depressed patients responded more rapidly to sad versus happy word targets, whereas manic patients displayed the opposite bias, responding faster to happy words.31
The patients with depression tend to ruminate over failures and criticism. Patients with depression also have an exaggerated response to negative feedback during the neuropsychological test of confrontation. An early study using two tests of working memory and forward planning reported that if patients with MDD responded incorrectly on a given trial, they were disproportionately likely to fail the subsequent trial.17 This exaggerated response to previous failure occurred across both tasks and could influence the cognitive ability on any tasks that deliver performance-contingent feedback. Moreover, this feedback sensitivity appeared specific to depression because it was not seen in other neuropsychiatric conditions that showed overall task impairments, such as Parkinson’s disease.32
In addition to abnormal processing of negative feedback in depression, the anhedonic symptoms of depression, wherein the patients fail to derive enjoyment from pleasurable activities, suggest that there may also be altered processing of positively valenced information in MDD. As such, anhedonia appears to reflect both a blunting of positive reinforcement processing, as well as an inability to use negative feedback to improve task performance.
Numerous imaging, postmortem, and laboratory studies showed diverse depression-related anatomy. Dorsal and lateral prefrontal cortex dysfunctions in functional imaging studies were found in depressed patients performing executive tasks. Hippocampal dysfunction is impaired during memory encoding tasks33 and reduced hippocampal volume is the most consistently reported neuropathological finding in MDD.27 Right orbitofrontal cortex and amygdala showed abnormally increased neural responses to sad targets and negative emotional faces.34 The amygdala is extensively interconnected with the multiple regions within the prefrontal cortex, and these interactions may allow top-down control of emotional behavior.
Biased cognitive processes may interact with genetic and neurobiological vulnerability factors.35 For example, variations in the serotonin transporter gene (5-HTTLPR) are known to be vulnerability factors for depression through their effects on social cognition.36 This gene-by-environment view of depression showed a recent integrative cognitive hypothesis which explains depression as the result of interplay among genes, neuroendocrine, and stress in relation to various cognitive biases.37
Although Alzheimer’s disease (AD) is a neurodegenerative disease characterized by impairment in various cognitive functions, depression is one of the most frequent accompanying psychiatric symptoms of AD, with 30% to 50% prevalence.38 The cognitive aspects of depression in patients with AD have been less studied compared to those of early-onset depression. Cognition is primarily and persistently affected during disease progression in AD, and according to the cognitive theory of depression, depression in AD may be associated with this impairment. In AD, the relationship between cognitive impairment and depression remains controversial. Some previous studies have reported about the negative impact of depression on general cognition,39 measures of dementia severity, working memory, processing speed,40 attention, motor functioning, visuospatial perception and construction.41 Other investigators have found no significant cognitive differences between AD patients with and without depression.42 Due to lack of a consistent relationship or a weak relationship between cognitive impairments and depression, it is still not clear whether depression is secondary to cognitive impairment or an epiphenomenon of AD.
Recent hospital-based studies in drug-naïve AD patients showed impairment on the digit forward, backward, calculation and controlled oral word association test compared to AD patients without depression. Moreover, specific cognitive neuropsychological tests and depression symptoms were significantly correlated.43 These findings suggested that these specific cognitive neuropsychological tests might be a state marker, in a dose-dependent manner. Studies of the use of antidepressants for depression in dementia are inconclusive, with several negative findings reported in recent large studies suggesting that antidepressant may not confer benefit over placebo. Interestingly, a recent retrospective donepezil study showed improvement in certain items of depression symptoms. These findings suggested heterogeneous symptomatology of depression.44
How does neurochemical disturbance cause someone to get depressed? Why restoring this chemical disturbance improves depression? Although neurochemical mechanisms of antidepressant drug action are of great interest, they do not provide an insight into the psychological mechanisms by which these neural changes help improve the depressed mood. From a psychological viewpoint, mood improvement has generally been regarded as the direct endpoint for antidepressants. However, this view has several drawbacks as drugs which act immediately and improve mood may not be clinically effective as antidepressants45 and drugs with an antidepressant action do not elevate mood in people who are not depressed.46
According to the newly formulated cognitive theory of depression, antidepressants work by redirecting negative affective biases in depression and these actions occur relatively quickly following drug administration. Although such cognitive changes are subtle in patients, the effects of processing emotional and social stimuli in a more positive manner would be expected to lead to gradual changes with accompanying social reinforcement, behavior and mood over time and experience of these cues. As described in Fig. 2, this view suggests that the critical time lag in antidepressant drug action does not result from a delay in relevant neuropharmacological actions, but is due to the time gap between the effects of antidepressants on cognitive bias change and the subsequent effects on mood. In other words, changes in affective bias with antidepressant drug administration do not directly enhance mood, but may provide a stepping-stone for subsequent cognitive and psychological reconsolidation. This view is consistent with cognitive theory of depression which emphasizes the role of negative biases in information processing in the development and relapse of depression and the importance of correcting such biases in successful treatment of this disorder.1
Depression is associated with cognition, and several domains of cognition (including hot cognition) are primarily affected. These cognitive dysfunctions may have a critical role in the development of depression and response to treatment. The modern cognitive theory of depression has been reformulated and expanded from the original cognitive model of depression based on the results from recent depression studies. This integrated approach had a profound effect on understanding the pathophysiology and treatment of depression.
According to the modern cognition theory of depression, future studies will benefit from integrative research in cognitive science which embraces the genetic, neural, cognitive, and affective aspects of depression.
Figures and Tables
References
1. Beck AT. Cognitive Therapy and the Emotional Disorders. 2nd ed. New York: International Universities Press;1976.
2. Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012; 37:117–136.

3. Boland RJ, Keller MB. Course and outcome of depression. In : Gotlib IH, Hammen CL, editors. Handbook of Depression. 2nd ed. New York: Guilford;2009. p. 23–43.
4. Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009; 195:102–108.

5. Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005; 66:455–468.

6. Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995; 117:285–305.

7. Ellenbogen MA, Schwartzman AE. Selective attention and avoidance on a pictorial cueing task during stress in clinically anxious and depressed participants. Behav Res Ther. 2009; 47:128–138.

8. Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005; 1:167–195.

9. Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008; 42:1118–1126.

10. Hammar A, Ardal G. Cognitive functioning in major depression--a summary. Front Hum Neurosci. 2009; 3:26.
11. Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004; 50:1–11.

12. Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry. 2002; 10:86–99.

13. Clark L, Sarna A, Goodwin GM. Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. Am J Psychiatry. 2005; 162:1980–1982.

14. Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007; 62:917–924.

15. Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996; 26:591–603.

16. Robbins TW. Dissociating executive functions of the prefrontal cortex. In : Roberts AC, Robbins TW, Weiskrantz L, editors. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford: Oxford University Press;1998. p. 117–130.
17. Elliott R, Baker SC, Rogers RD, O’Leary DA, Paykel ES, Frith CD, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997; 27:931–942.

18. Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology. 2003; 47:21–26.

19. Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehéricy S, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005; 26:860–869.

20. Hugdahl K, Rund BR, Lund A, Asbjørnsen A, Egeland J, Ersland L, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004; 161:286–293.

21. Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel HJ, et al. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry. 2006; 59:958–965.

22. Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry. 2007; 164:516–519.

23. Gorwood P, Corruble E, Falissard B, Goodwin GM. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry. 2008; 165:731–739.

24. Martinez-Aran A, Vieta E, Torrent C, Sanchez-Moreno J, Goikolea JM, Salamero M, et al. Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar Disord. 2007; 9:103–113.

25. Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004; 161:598–607.

26. Deckersbach T, Dougherty DD, Savage C, McMurrich S, Fischman AJ, Nierenberg A, et al. Impaired recruitment of the dorsolateral prefrontal cortex and hippocampus during encoding in bipolar disorder. Biol Psychiatry. 2006; 59:138–146.

27. Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004; 56:640–650.

28. Matt GE, Vázquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: a meta-analytic review. Clin Psychol Rev. 1992; 12:227–255.

29. Brittlebank AD, Scott J, Williams JM, Ferrier IN. Autobiographical memory in depression: state or trait marker? Br J Psychiatry. 1993; 162:118–121.

30. Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002; 159:302–304.

31. Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999; 29:1307–1321.

32. Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry. 1997; 63:74–82.

33. Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry. 2004; 161:637–645.

34. Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008; 63:377–384.

35. De Raedt R, Koster EH. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci. 2010; 10:50–70.

36. Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011; 69:513–519.

37. Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011; 12:467–477.

38. Kwak YT, Yang Y, Koo MS. Depression in Alzheimer’s disease. Dement Neurocogn Disord. 2014; 13:27–36.

39. Rovner BW, Broadhead J, Spencer M, Carson K, Folstein MF. Depression and Alzheimer’s disease. Am J Psychiatry. 1989; 146:350–353.

40. Rubin EH, Kinscherf DA, Grant EA, Storandt M. The influence of major depression on clinical and psychometric assessment of senile dementia of the Alzheimer type. Am J Psychiatry. 1991; 148:1164–1171.

41. Wefel JS, Hoyt BD, Massma PJ. Neuropsychological functioning in depressed versus nondepressed participants with Alzheimer’s disease. Clin Neuropsychol. 1999; 13:249–257.

42. Lopez OL, Boller F, Becker JT, Miller M, Reynolds CF 3rd. Alzheimer’s disease and depression: neuropsychological impairment and progression of the illness. Am J Psychiatry. 1990; 147:855–860.

43. Yang Y, Kwak YT. The neuropsychological characteristics in early stage of Alzheimer’s patients with depression. Dement Neurocogn Disord. 2016; 15:37–42.

44. Yang Y, Kwak YT. The effects of donepezil on 15-item geriatric depression scale structure in patients with Alzheimer disease. Dement Geriatr Cogn Dis Extra. 2016; 6:437–446.

45. Satel SL, Nelson JC. Stimulants in the treatment of depression: a critical overview. J Clin Psychiatry. 1989; 50:241–249.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download