Abstract
Background
The dot-like hippocampal signal intensity in diffusion-weighted MR images is well-known as a characteristic imaging feature in transient global amnesia, a neurological syndrome in which sudden forward-and-backward memory loss occurs that is slowly recovered within 24 hours. We here report on patients with this dot-like hippocampal hyperintensity who did not present with anterograde amnesia except for headaches.
Case Report
Two women without a specific medical history presented with sudden-onset headaches on the same day. Neither had any trauma or infection history before the symptom or any sudden emotional or postural changes. Brain MRI showed tiny hippocampal high signal intensity on diffusion-weighted images (DWI).
The dot-like hippocampal signal intensity in diffusion-weighted images (DWI) is well-known as a characteristic imaging feature in transient global amnesia (TGA).1 It is seen in single or multiple lesions that range in size from 1 mm to 5 mm and can be seen in both the unilateral and bilateral hippocampus.2 TGA is a neurological syndrome in which sudden forward-and-backward memory loss occurs that is slowly recovered within 24 hours.3 We here report on two patients with these dot-like hippocampal hyperintensities who did not present with anterograde amnesia except for headaches.
A 65-year-old woman without a specific medical history presented with a sudden onset of headache one day; the headache had developed suddenly while the patient had been watching TV, and the headache area was confined to the occiput. She had experienced no trauma or infection history before the symptom and no sudden emotional or postural changes as she watched TV. The pattern of headache was non-pulsatile, and it was the first headache the patient had experienced. The pain intensity was not sufficient to interfere with daily life, and she had no accompanying symptoms such as nausea, vomiting, or dizziness. At the time of admission, the patient's physical signs were normal, and her neurological examination revealed no specific findings. One week later, the patient revisited the clinic. Headache intensity had not improved, so we decided to conduct additional examination to differentiate the secondary headache. There were no abnormal findings on the baseline blood test or electrocardiogram, but brain MRI showed dot-like hippocampal signal intensity on DWI with matching low apparent diffusion coefficient values; there were no other brain lesions on T2-weighted images, and cerebral blood vessels were normal (Fig. 1). In a detailed discussion, the patient described all the events in chronological order with no deterioration in cognitive function including memory. The patient's headache relieved gradually with administration of tricyclic antidepressants, and she is currently being followed up with no headache recurrence.
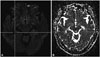
This patient visited the hospital with a headache that had started one week before. The headache started suddenly while she was washing, a pain that started at the back of the neck and spread around the eyes. The character of headache was non-pulsatile, and it was not a pain the patient had experienced before. The headache was most painful within a few minutes, but it had gradually improved at the time of her visit; she had no associated symptoms. In the clinic, the patient's physical signs were normal, and we found no specific findings on neurologic examination. The patient showed no cognitive impairment, including memory, when she and her caregiver were interviewed. Because the patient's headache had a thunderclap nature, we performed MRI to differentiate secondary headache. Her brain DWI showed tiny high signal intensity in the right hippocampus (Fig. 2), but we observed no aneurysms or stenosis on cerebral angiography and no white degeneration or lacunar infarction on T2-weighted images. The patient's headache improved on its own with no medication, and we are following her with no recurrence.
TGA is a syndrome characterized by prospective memory impairment that disappears within 24 hours;4 its diagnosis is based on characteristic clinical features.5 In addition, DWI will show high signal intensity in the hippocampus in most patients from 24 hours to 72 hours after symptom onset, and these image findings are known to characterize TGA.6 Usually, the hippocampal lesions seen on DWI distinguish TGA from cerebral infarction, infection, and so on; however, dot-like lesions are rarely seen in other diseases (Table 1).1 In general, TGA can be accompanied by characteristic anterograde amnesia and other symptoms, the most common being headache.7 We searched the literature about the relationship between headache and hippocampal lesions, but we found no similar cases. Migraine is a well-known risk factor for TGA,8 but we did not diagnose this case as migraine because the headache's features were different from migraine.
Two recent papers have been published for patients with dot-like hippocampal lesions but no prospective memory impairment. Foster et al.9 compared 12 TGA patients who had dot-like hippocampal lesions with 10 cerebral infarct patients who showed isolated punctuate hippocampal lesions. The authors asserted that DWI alone cannot distinguish between TGA and cerebral infarction and suggested that the accompanying neurological symptoms and clinical features are important because neurologic abnormalities such as vertigo and dysarthria were more frequently observed in the cerebral infarction group. In contrast, Jeong et al.10 reported eight patients with dot-like hippocampal lesions without anterograde amnesia, and these lesions can be seen in patients with a history of Valsalva manipulation. Kim et al. argued that this phenomenon was attributable to individual sensitivity to hippocampal lesion manifestations on Valsalva manipulation (Table 2).
In this case, the absence of accompanying neurologic abnormalities is explained by a mechanism similar to that of TGA, but the absence of Valsalva manipulation requires a differential diagnosis for cerebral infarction and other diseases. One unusual thing is that both published papers described accompanying symptoms, but no patients had accompanying headaches. This patient in our study is thought to be the first to show dot-like hippocampal lesions on DWI with only headache and no symptoms of TGA.
The pathophysiology of TGA is still hypothesized to be hemodynamic abnormalities such as cerebral infarction, diffusion of cortical inhibition, or venous congestion; of these, venous congestion is the most likely.111213 If veins are clogged, intravenous hypertension increases the risk of venous congestion and subsequent ischemic injury,14 although if these ischemic injuries are not sufficiently severe to cause memory impairment, clinicians may only observe imaging findings without memory disturbances, as in this case. However, whether venous congestion can cause headache or whether the stress caused by headache causes hippocampal lesions is still a question that needs to be resolved.
In conclusion, dot-like hippocampal lesions seen on DWI may be present without memory impairment, and additional studies are needed to determine whether there is any association with headache as in case 2 in this study.
Figures and Tables
Fig. 1
Hippocampal hyperintensity in diffusion-weighted imaging in case 1. A: Diffusion-weighted images. B: Apparent diffusion coefficient.
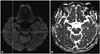
Fig. 2
Hippocampal hyperintensity in diffusion-weighted imaging in case 2. A: Diffusion-weighted images. B: Apparent diffusion coefficient.
References
1. Förster A, Griebe M, Gass A, Kern R, Hennerici MG, Szabo K. Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovasc Dis. 2012; 33:104–115.

2. Weon YC, Kim JH, Lee JS, Kim SY. Optimal diffusion-weighted imaging protocol for lesion detection in transient global amnesia. AJNR Am J Neuroradiol. 2008; 29:1324–1328.

3. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010; 9:205–214.

4. Kritchevsky M, Zouzounis J, Squire LR. Transient global amnesia and functional retrograde amnesia: contrasting examples of episodic memory loss. Philos Trans R Soc Lond B Biol Sci. 1997; 352:1747–1754.

5. Quinette P, Guillery-Girard B, Dayan J, de la Sayette V, Marquis S, Viader F, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006; 129(Pt 7):1640–1658.

6. Bartsch T, Alfke K, Stingele R, Rohr A, Freitag-Wolf S, Jansen O, et al. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 2006; 129(Pt 11):2874–2884.

8. Donnet A. Transient global amnesia triggered by migraine in a French Tertiary-Care Center: an 11-year retrospective analysis. Headache. 2015; 55:853–859.

9. Förster A, Al-Zghloul M, Wenz H, Böhme J, Groden C, Neumaier-Probst E. Isolated punctuate hippocampal infarction and transient global amnesia are indistinguishable by means of MRI. Int J Stroke. 2017; 12:292–296.

10. Jeong M, Jin J, Kim JH, Moon Y, Choi JW, Kim HY. Incidental hippocampal hyperintensity on diffusion-weighted MRI: individual susceptibility to transient global amnesia. Neurologist. 2017; 22:103–106.
11. Frisoni GB. PET and 18F ligands in the diagnosis of Alzheimer's disease. Lancet Neurol. 2011; 10:397–399.

12. Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol Scand. 2000; 102:275–283.

13. Menéndez González M, Rivera MM. Transient global amnesia: increasing evidence of a venous etiology. Arch Neurol. 2006; 63:1334–1336.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download