This article has been
cited by other articles in ScienceCentral.
Abstract
Background
An infection known to be a major cause of mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Rapidly progressive dementia is a neurological condition in which dementia progresses in a short period of time.
Case Report
We report on a 78-year-old woman presenting with a rapid decline in cognitive function resulting from a scrub typhus infection. Diffusion weighted images showed a signal intensity at the splenium, and subcortical white matter of both hemispheres suggesting MERS. On the neuropsychological test, the patient showed frontal executive dysfunction.
Conclusions
This case suggests that diagnosticians should consider the possibility that a MERS patient with a rapidly cognitive decline could have a scrub typhus infection because early diagnosis of scrub typhus is very important in this aspect of the treatment.
Go to :

Keywords: splenial lesion, dementia, scrub typhus
INTRODUCTION
Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is a clinico-radiological entity presenting with various clinical symptoms and signs, including change of consciousness, seizure, or headache.
1 Transient abnormalities in the corpus callous, especially the splenium, in neuroimaging is a characteristic finding of MERS. Infectious agents have been known to be the major cause of MERS.
2
Scrub typhus implicated by
Orientia tsutsugamushi is one of the causes of acute febrile illness in the Asia-Pacific region.
3 Clinical manifestations of scrub typhus could include fever, lymphadenopathy, abnormal liver function, acute respiratory distress syndrome, CNS involvement, septic shock, and so on.
45 Neurologic manifestations caused by scrub typhus include an altered mentality, seizures, encephalopathy, focal neurologic deficit, myelitis, meningoencephalitis, and so on.
6
Rapidly progressive dementia (RPD) is referred to as a neurological condition in which dementia progresses less than 1–2 years from onset.
7 Moreover, RPD could develop subtly over months, weeks or even days while most dementia conditions caused by neurodegenerative disorders develop gradually over years.
89 Several etiologies including prion diseases, degenerative conditions, autoimmune disorders, vascular disease, and infection have been known to be the causes of RPD.
8910 Considering that some patients are potentially treatable for RPD, early identification of causes for RPD is essential in certain aspects of the prognosis and the choice of drugs to utilize in treating this disease.
To our knowledge, there has been no report for MERS caused by scrub typhus. We herein report a 78-year-old woman who had MERS caused by a scrub typhus infection presenting as RPD.
Go to :

CASE REPORT
A 78-year-old women visited our emergency department in October due to behavioral changes and emotional instability which had commenced 2 days prior. Just before her hospital visit, the patient had walked around the village barefoot without purpose. The patient had no past medical history including diabetes, hypertension, liver disease, mental illness and so on. The patient had worked in urban areas as a farmer without any prior illness.
The body temperature of the patient was 38.3℃. Her blood pressure and pulse rate were 130/80 mm Hg and 90 beats/min, respectively. There were no skin lesions such as eschar or a rash. During the initial neurological examination, the patient had impaired concentration, decreased speech capabilities, and was disorientated concerning the time, place, and person. The patient was an elementary school graduate. There was no localized abnormal neurologic signs of cranial nerves, motor, and sensory examination.
The initial laboratory tests included a complete blood count, blood chemistry, HIV antibody, influenza antibody, Orienta tsutsugamushi antibody, leptospira antibody, Hanta virus antibody, hepatitis A, B, and C antibody using immunofluorescent assay; most were within normal limits except for an elevated C-reactive protein (6.8 mg/dL: normal range was below 0.5 mg/dL). The cerebrospinial fluid (CSF) examination also showed results within normal limits; the opening pressure was 15 cm H2O with a leukocyte count of 0/mm3, a protein level of 40 mg/dL, a glucose level of 81 mg/dL (serum glucose level of 115 mg/dL). The polymerase chain reaction for herpes simplex virus, tuberculosis of CSF had a negative result. Microbiologic assays including Gram stain, acid fast bacilli staining, indian ink stain, and cultures for CSF and blood were negative result. An oligoclonal band was not identified on the electrophoresis. The EEG did not show epileptiform discharge except for a mild amount of high amplitude arrhythmic delta activity.
A conventional MRI using a 1.5-T magnetic resonance scanner (Gyroscan, Philips, Best, the Netherlands) was performed on admission. It showed bilateral white matter hyperintensities on a T2-weighted image which was considered as common incidental findings of clinically healthy subjects with age (not shown). There was no abnormal findings in the Gadolinium contrast enhancement sequence. However, the diffusion weighted image (DWI:
b-value=1000 s/mm
2) scan showed a high signal intensity at the splenium and subcortical white matter of both hemispheres (
Fig. 1A, B, D, and E).
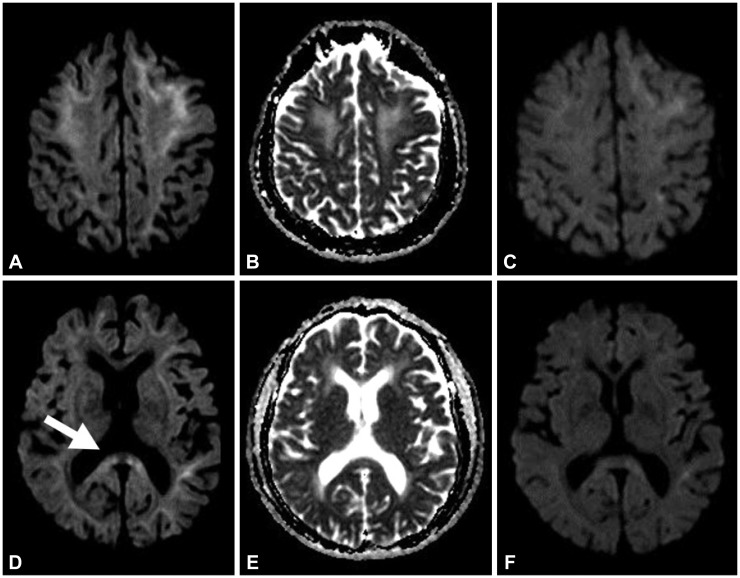 | Fig. 1Initial DWI scan showed high signal intensity at splenium (white arrow) and subcortical white matter of both hemispheres (A, B, D, and E). In follow-up DWI performed after 2 months later, the abnormal signal intensity in splenium and subcortical white matter was improved, but a slight abnormality remained (C and F). DWI: diffusion weighted image.
|
Three days after admission, the patient suddenly developed dyspnea. A chest X-ray showed diffuse infiltration in both lung parenchyma. The patient's fever was not improved. On a follow-up analysis of the serum, there were thrombocytopenia, leukocytosis, increased aspartate aminotransferase, and alanine aminotransferase to 108 IU/L and 96 IU/L, respectively.
Empirical antibiotics with ceftriaxone, azithromycin, and doxycycline were given. Two days later, the patient rapidly improved. The fever subsided. The profile of the complete blood count, liver function test, and chest X-ray were normalized. The follow-up analysis of scrub typhus antibody tilters against Orientia tsutsugamushi were increased to 1:540, which enabled us to confirm a diagnosis of scrub typhus. After two weeks had passed, the patient was discharged to go home although both the patient and her caregiver complained of cognitive decline and personality changes including violent behavior, emotional blunting, compulsive eating, and so on.
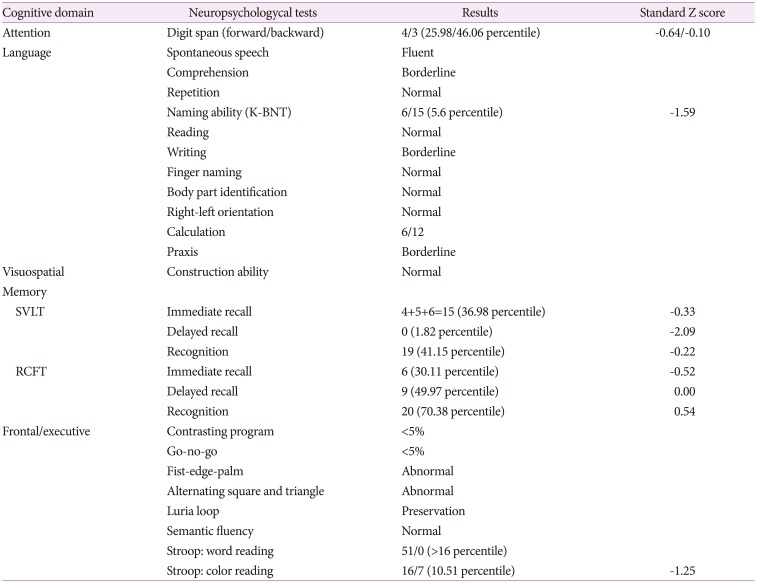
After a month later, the neuropsychological test was performed: The Korean version of the Mini Mental Status Examination score was 19/30. The patient showed lower scores, especially on her frontal executive function. The performance on the contrasting program, the Go-No Go test, the Stroop test color reading, the fist-edge-palm, alternating square and triangle and Luria loop fell below the normal limits. In language and related function, a confrontational naming score had declined with borderline abnormality in comprehension, writing, calculation, and praxis (
Table 1).
Table 1
Neuropsychological data of the patient

In a follow-up DWI performed 2 months later, the abnormal signal intensity in the splenium and the subcortical white matter was improved, but a slight abnormality remained (
Fig. 1C and F). There was a slight improvement of cognitive dysfunction in the patient. However, the patient's level of dimentia did not return to her pre-hospitalalization condition.
Go to :

DISCUSSION
The interesting findings of our patient were as follows: first, the patient suffered from a non-systemic illness but also CNS involvement by scrub typhus. CNS involvement of infectious organisms including herpes virus, HIV, toxoplasmosis,
Cryptococcus, cytomegalovirus, syphilis,
Borrelia, cysticercosis could be a cause of dementia.
11 There have been several reports of meningitis and encephalitis caused by scrub typhus in endemic areas, especially in the Asian-Pacific regions.
5 Several studies have demonstrated that the incident rate of infection during the last few decades had been rapidly increasing in Korea.
121314 Moreover, climate change may have had an effect on the incident rate and its acceleration.
15 Although the neurologic manifestations caused by scrub typhus resemble aseptic meningitis or encephalitis, a focal neurologic deficit or sign caused by it has proven to be rare.
16 The CSF analysis of our patient was not compatible with the findings of meningitis like in other reports.
1617 Instead, the findings of the DWI suggest MERS with extra-callosal involvement.
18 The exact pathophysiological mechanism of MERS remains to be seen. Considering scrub typhus involves the vascular endothelium,
19 vasculitis might be associated with MERS in our patient.
Second, the patient had RPD after a scrub typhus infection. Although we did not have previous information about cognitive function, the RPD of our patient must result from a scrub typhus infection based on history, clinical manifestations, the neuroimaging, and the neuropsychologic tests, which were performed a month after treatment of the infection. The neuropsychologic test of our patient showed frontal dysfunction, which could be compatible with the neuroimaging findings concerning the high signal change of the DWI. The cognitive decline and behavioral changes, even after treatment of the scrub typhus infection, could imply that the brain damage resulting from it might be irreversible although the abnormal signal in DWI improved.
The limitations of this case is that there was no cognitive information about the patient before hospitalization. So, it was not possible to deduce objectively how rapidly the cognitive decline of the patient had progressed and persisted.
In summary, this case suggests that, in endemic areas, a MERS patient with febrile illness should be considered as possibly having a scrub typhus infection because the early diagnosis of scrub typhus is very important in this aspect of the treatment.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download