Abstract
Background and Purpose
Torrance Tests of Creative Thinking (TTCT) is a well-known and commonly used measure of creativity. However, the TTCT-induced creative hemodynamic brain activity is rarely revealed. The purpose of this study is to elucidate the neural correlates of creative thinking in the setting of a modified version of the figural TTCT adapted for an functional magnetic resonance imaging (fMRI) experiment.
Methods
We designed a blocked fMRI experiment. Twenty-five participants (11 males, 14 females, mean age 19.9±1.8) were asked to complete the partially presented line drawing of the figural TTCT (creative drawing imagery; creative). As a control condition, subjects were asked to keep tracking the line on the screen (line tracking; control).
Results
Compared to the control condition, creative condition revealed greater activation in the distributed and bilateral brain regions including the left anterior cingulate, bilateral frontal, parietal, temporal and occipital regions as shown in the previous creativity studies.
Conclusions
The present revealed the neural basis underlying the figural TTCT using fMRI, providing an evidence of brain areas encompassing the figural TTCT. Considering the significance of a creativity test for dementia patients, the neural correlates of TTCT elucidated by this study may be valuable to evaluate the brain function of patients in the clinical field.
Creativity refers to the ability to produce something novel and useful,1 that can be measured by many aspects such as divergent thinking and free association using various tests.23 As one of the divergent thinking production tests for creativity, the Torrance Tests of Creative Thinking (TTCT)4 is one of the most well-known and commonly used batteries.5 The TTCT is highly recommended in education and business fields.6 In addition, it is suggested to be useful in the clinical field for testing the ability of divergent and flexible thinking in dementia patients.7
In recent years, major advances in neuroimaging technique enables us to elucidate brain areas involving specialized cognitive function such as creative thinking. Some studies using neuroimaging tools such as functional magnetic resonance imaging (fMRI) and electroencephalography have reported brain areas or networks for creativity (see reviews by238). Most of the imaging study for creativity have employed experimental tasks by using Alternative Uses Test and Remote Associates Test, but the studies using the TTCT are rare.3
In this study, we examined brain areas serving TTCT performance using an fMRI. TTCT consisted of two subsets, verbal and figural form. In particular, the figural TTCT has been revealed to be fair in terms of gender, culture, and language, socio-economic status, and cultural background,9 so this study used the figural TTCT. We modified a paper-and-pencil test of the figural TTCT for an fMRI scanner to measure hemodynamic response in cortical regions engaged in figural TTCT performance. One of the figural TTCT items was presented for 30 s in MRI scanner, while participants were instructed to complete the given partial drawing in their mind. To capture only the cognitive process for figural TTCT, we controlled sensory processing by comparing brain activation during tracking the given stimulus. We hypothesized that creative thinking of the figural TTCT is served by bilateral brain regions including the prefrontal, parietal regions as other visuospatial divergent thinking production tasks.1011 This study will support the neural basis of creative thinking induced by figural TTCT.
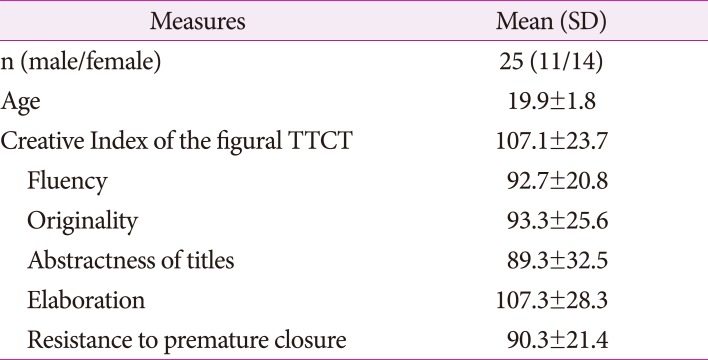
Twenty-five students (11 males, 14 females, mean age 19.9±1.8, range: 18–24) of Teachers' College at Dongguk University, Seoul, Korea, enrolled in this study. Prior to the scanning session, participants' creativity was evaluated based on the composite score of the parallel form A of the figural TTCT. The composite score of the test was called a Creative Index (CI) that is defined by sum of the averaged standard scores of the five subscales (i.e., fluency, originality, abstractness of titles, elaboration, and resistance to premature closure) and creative strength.4 The participants' mean CI of the figural TTCT form A was 107.1±23.7 (range: 67–141). The mean and standard deviation of each subscales were shown in Table 1. Participants were right-handed assessed by Edinburgh Handedness Inventory12 and no one had the following states: previous neurological illness, history of learning disability, head trauma with loss of consciousness, current or past use of psycho stimulant medications, or pregnancy. This study was approved by the Institutional Review Board for Public Authority specified by Korean Ministry of Health and Welfare. After complete description of the study, all volunteers signed a written informed consent.
This study used the figural TTCT to measure the creative thinking. The figural TTCT has two parallel forms, A and B and comprises three activities: picture construction, picture completion, and completion of repeated figures of lines or circles.6 Among the activities, the picture completion was selected as an active condition and referred to as creative drawing imagery.
The parallel form A of the figural TTCT was used for screening the participants before scanning while form B was presented during the fMRI experiment. For the fMRI experiment, 12 line drawings were used for task conditions. Six of the line drawings were from the figural TTCT form B and only used for the creative drawing imagery (creative condition). The other six line drawings were devised as control stimuli and used only for the line-tracking (control condition). For each condition, the stimuli were counterbalanced across subjects.
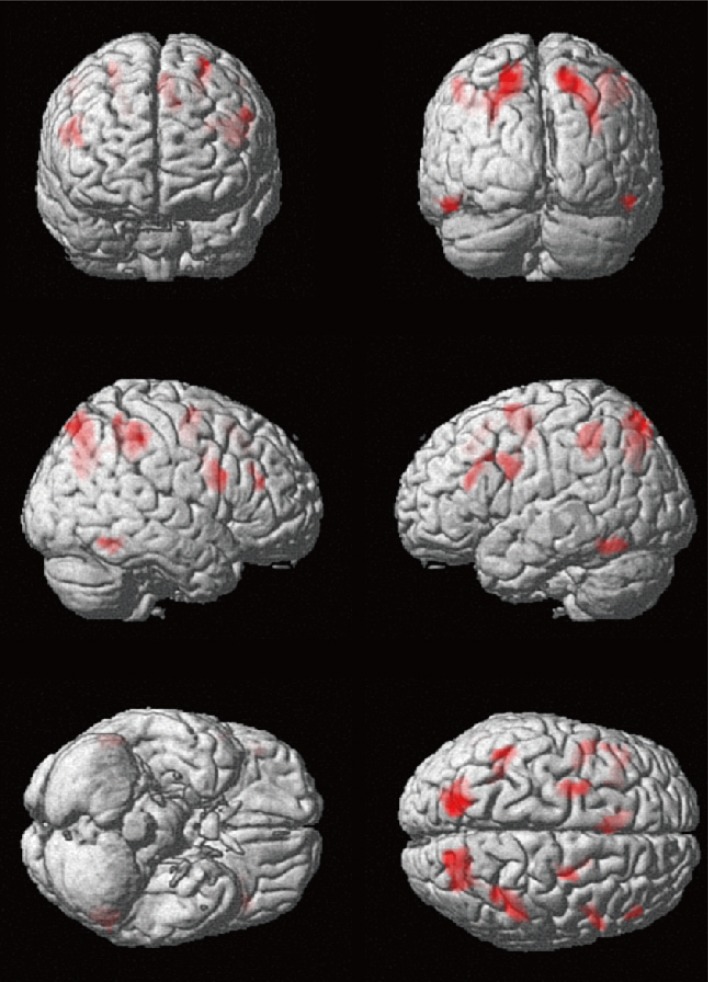
The experiment was composed of two sessions: the scanning and recall session. In the scanning session, participants performed a task while lying in the MRI scanner. There were 12 trials and each trial consisted of three periods: an instruction, a fixation, and an item period (Fig. 1). A trial began with an instruction shown for 7 s and subsequently fixation was presented for 3 s and an item for 30 s in turn. During the item presentation, subjects were instructed to either perform creative imagery or line-tracking according to the instruction. In fact, to adapt the figural TTCT for fMRI experiment, we abridged the original 10-minute activity of the figural TTCT to 30 seconds. In the creative condition, participants were instructed to complete the partially given line drawing in their mind, while in the control condition, participants were directed to keep tracking the given line with their eyes. Each condition was composed of six trials and arranged with alternative manner. All trials were presented using the E-Prime software (Psychology Software Tools, Pittsburgh, PA, USA) and projected on a screen behind the scanner. Participants viewed the visual stimuli through a mirror attached to a head coil. After completion of the scanning session, participants were asked to recall their complete figures outside of the scanner. This test session allowed confirming the compliance of the task of the participants.
The imaging was done by the 3T Magnetom Skyra MRI scanner (Siemens, Munich, Germany) at Dongguk University Ilsan Hospital, Goyang, Korea. The echo planar imaging (EPI) pulse sequence parameters were repetition time (TR)/echo time (TE)=3000/30 ms; field of view (FOV)=200 mm2; flip angle=90°; spatial resolution=2.0×2.0×5.0 mm. Participants were instructed not to move and avoid moving their heads during the scanning session. In addition, a high-resolution T1-weighted 3-dimensional (3D) anatomical scan was obtained using 3D magnetization-prepared rapid gradient-echo sequence protocol with the parameters as follows: TR/TE=2300/2.29 ms; FOV=240 mm2; flip angle=80°; spatial resolution=0.9×0.9×0.9 mm.
FMRI data analysis was conducted using Statistical Parametric Mapping (SPM) 8 toolbox (Wellcome Trust Centre for Neuroimaging, London, UK). Brain slices were realigned for motion correction, and T1-weighted anatomical images were co-registered to the mean EPI using a normalized mutual information. Subsequently, the spatial normalization parameter was estimated while the co-registered T1-weighted image was normalized to Montreal Neurological Institute (MNI) standard space by an affine and nonlinear algorithm, which was applied to EPIs. The normalized EPIs were smoothed with a Gaussian kernel of four mm full-width at half maximum to enhance the signal to noise ratio.
In the first-level statistical analysis (i.e., individual analysis), epoch-related blood oxygen level dependent response was modeled by box-car function with the convolution of canonical hemodynamic response function. Task-related brain activation was identified with the contrast of experimental condition>control condition epoch. To identify the creativity-related brain activation, the second-level analysis (i.e., group analysis) was carried out. The resulting activations were found at p<0.05 after family-wise error (FWE) correction for multiple comparisons, and observed at p<0.001 with a minimum extent threshold of 50 contiguous voxels without correction for multiple comparisons. The threshold activation was overlaid on the 3D-brain image by projecting them onto the surface of the brain using SPM. Anatomical regions of the peak that survived were labeled using Talairach Client1314 after converting coordinates from MNI to Talairach space using icbm2tal transform.15
Group analysis of creative condition compared to control condition revealed two contiguous voxels in the right temporal regions, specifically fusiform gyrus (FWE corrected p<0.05) (Table 2, Fig. 2). To observe brain areas involved in creative imagery, we set a less conservative threshold at uncorrected p<0.001 and found multiple brain areas of bilateral hemispheres including the right inferior frontal and bilateral middle frontal, precentral regions and the left superior frontal regions, and bilateral cingulate, precuneus and fusiform gyrus (Table 2, Fig. 2). The findings indicate that the figural TTCT performance is executed by distributed brain regions, not by single region or a certain dominant hemisphere, as revealed by other visuospatial creative tasks based on divergent thinking production.
In this study, we investigated brain regions involved in the TTCT, the most commonly used creativity test, using fMRI. As expected, group analysis revealed several brain areas in frontal, temporal, parietal, and occipital area activated during the figural form of the TTCT than during tracking the stimuli. Our findings are supportive of multiple and bilateral involvement in the brain for creative thinking, which is in accordance with previous findings (see reviews310).
Significant brain activation was revealed in the right fusiform gyrus. The right fusiform gyrus has been suggested to engage in visual imagery,1617 which is plausible since the task is an imagery task. This visual imagery region has stronger functional connectivity with precuneus, as a part of default mode network, when compared brain activation in artist to in non-artist during visual creativity tasks (i.e., planning an artwork).18 They suggested this as the divergent thinking and producing novel ideas for creative thinking. In our study, precuneus was also activated bilaterally. Although we did not perform functional connectivity analysis among activated regions, two regional significant activation may indicate possible functional connectivity for creative thinking.
The resulting brain areas also included anterior cingulate regions covering Brodmann area (BA)24 and BA32, which is suggested to be modulated by the attention-demanding task requiring attention and effortful thoughts.19 In the creative drawing imagery task, participants produce novel ideas and elaborate them, which might need more attention and cognitive loading and yield more activation in the anterior cingulate gyrus than simple line-tracking condition.
Additionally, we found greater brain activation in bilateral middle frontal regions during creative drawing imagery than line-tracking. These activated clusters belong to BA46, BA9, and BA8, that are parts of the dorsolateral prefrontal cortex (DLPFC). The activation of DLPFC is in line with the previous study on creativity using the figural TTCT20 and another visuospatial creative task based on divergent thinking.21 As suggested in the previous finding, the DLPFC is suggested to play a role in production of novel responses,22 goal-related visual search,23 focused attention,24 analogic reasoning.25 These functions may be critical for visual creativity.
In addition, BA6 is revealed to be activated during mental operation task,26 which fits our creative task where volunteers were instructed to perform only in their mind. Bilateral temporal region of BA37 was also engaged, which is suggested to be a common node of language and visual perception.27 It is also observed that the BA37 is co-activated with the left prefrontal region including BA9 and the right precuneus (BA7). BA9 engages in language control, verbal fluency and verbal reasoning,2829 while the right parietal precuneus (BA7) contribute to visuospatial representation.30 This network may support creative imagery by providing semantic and linguistic information of visuospatial imagination.
Our findings revealed that neural correlates involved in figurative creative thinking were in bilateral and multiple brain regions including visual imagery, visuospatial presentation, attention, fluency and originality. Considering dementia patients suffer from cognitive deterioration such as visuospatial function and attention, fluency, etc., it would be of considerable interest to investigate creativity in dementia patients. Previous behavioral studies revealed the inability of patients with Alzheimer's disease to engage in creative thinking when generating novel object drawings and they tend to produce more perseverative errors than controls.31 In a future neuroimaging study on creativity in dementia, these behavioral abnormalities may be represented as deactivation in the appropriate brain regions. In addition, creative activity is suggested to be a good rehabilitation strategy for dementia patients since creativity can regulate the emotional processing.7 Its therapeutic effect might be explained in the brain regions found in this study.
In conclusion, this study examined the neural basis underlying the figural TTCT in the setting of an fMRI, providing evidence of brain areas involved in the figural TTCT. Considering a lack of study on task-evoked cortical areas for the TTCT in spite of growing interest in the use of the TTCT in clinical field,7 this study provides supportive evidence of the neural correlates of the TTCT.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2013S1A3A2042899).
References
2. Fink A, Benedek M, Grabner RH, Staudt B, Neubauer AC. Creativity meets neuroscience: experimental tasks for the neuroscientific study of creative thinking. Methods. 2007; 42:68–76. PMID: 17434417.

3. Arden R, Chavez RS, Grazioplene R, Jung RE. Neuroimaging creativity: a psychometric view. Behav Brain Res. 2010; 214:143–156. PMID: 20488210.

4. Torrance EP.Torrance Tests of Creative Thinking: norms-technical manual: figural (streamlined) forms A & B. Bensenville: Scholastic Testing Service;1998.
5. Piffer D. Can creativity be measured? An attempt to clarify the notion of creativity and general directions for future research. Think Skills Creat. 2012; 7:258–264.

6. Kim KH. Can we trust creativity tests? A review of the Torrance Tests of Creative Thinking (TTCT). Creat Res J. 2006; 18:3–14.

7. Palmiero M, Di Giacomo D, Passafiume D. Creativity and dementia: a review. Cogn Process. 2012; 13:193–209. PMID: 22438178.

8. Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull. 2010; 136:822–848. PMID: 20804237.

9. Cramond B. The Torrance Tests of Creative Thinking: from design through establishment of predictive validity. In : Subotnik RF, Arnold KD, editors. Beyond terman: contemporary longitudinal studies of giftedness and talent. Norwood: Ablex;1994. p. 229–254.
10. Wiggins GA, Bhattacharya J. Mind the gap: an attempt to bridge computational and neuroscientific approaches to study creativity. Front Hum Neurosci. 2014; 8:540. PMID: 25104930.

12. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9:97–113. PMID: 5146491.

13. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000; 10:120–131. PMID: 10912591.

14. Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997; 5:238–242. PMID: 20408222.

15. Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007; 28:1194–1205. PMID: 17266101.

16. Mechelli A, Price CJ, Friston KJ, Ishai A. Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb Cortex. 2004; 14:1256–1265. PMID: 15192010.

17. Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res. 2004; 20:226–241. PMID: 15183394.

18. De Pisapia N, Bacci F, Parrott D, Melcher D. Brain networks for visual creativity: a functional connectivity study of planning a visual artwork. Sci Rep. 2016; 6:39185. PMID: 27991592.

19. Davis KD, Hutchison WD, Lozano AM, Tasker RR, Dostrovsky JO. Human anterior cingulate cortex neurons modulated by attention-demanding tasks. J Neurophysiol. 2000; 83:3575–3577. PMID: 10848573.

20. Huang P, Qiu L, Shen L, Zhang Y, Song Z, Qi Z, et al. Evidence for a left-over-right inhibitory mechanism during figural creative thinking in healthy nonartists. Hum Brain Mapp. 2013; 34:2724–2732. PMID: 22522783.

21. Aziz-Zadeh L, Liew SL, Dandekar F. Exploring the neural correlates of visual creativity. Soc Cogn Affect Neurosci. 2013; 8:475–480. PMID: 22349801.

22. Jahanshahi M, Dirnberger G. The left dorsolateral prefrontal cortex and random generation of responses: studies with transcranial magnetic stimulation. Neuropsychologia. 1999; 37:181–190. PMID: 10080375.

23. Pollmann S, von Cramon DY. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Exp Brain Res. 2000; 133:12–22. PMID: 10933206.

24. Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006; 16:1679–1689. PMID: 16436686.

25. Boroojerdi B, Phipps M, Kopylev L, Wharton CM, Cohen LG, Grafman J. Enhancing analogic reasoning with rTMS over the left prefrontal cortex. Neurology. 2001; 56:526–528. PMID: 11222799.

26. Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, et al. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb Cortex. 2002; 12:1157–1170. PMID: 12379604.

27. Ardila A, Bernal B, Rosselli M. Language and visual perception associations: meta-analytic connectivity modeling of Brodmann area 37. Behav Neurol. 2015; 2015:565871. PMID: 25648869.

28. Luria AR. Basic Problems of Neurolinguistics. Hague: Walter de Gruyter;1976.
29. Berthier ML. Transcortical Aphasias. Hove: Psychology Press;1999.
30. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006; 129(Pt 3):564–583. PMID: 16399806.

31. Bigler ED. Design fluency in dementia of Alzheimer’s type, multiinfarct dementia and dementia associated with alcoholism. Appl Neuropsychol. 1995; 2:7–14. PMID: 16318546.

Fig. 1
fMRI experimental paradigm for creative thinking using the figural TTCT. A modified figural TTCT was used to examine brain regions related to visual creativity using fMRI. fMRI experiments consisted of the two conditions: creative drawing imagery (creative) as an active condition and line-tracking (control) as a control condition. For each condition there were six trials, where an instruction was presented for 7 s, a fixation for 3 s, and an item for 30 s subsequently. The items for creative condition were from the figural TTCT form B while that for control condition were novel items devised for this study. fMRI: functional magnetic resonance imaging, TTCT: Torrance Tests of Creative Thinking.
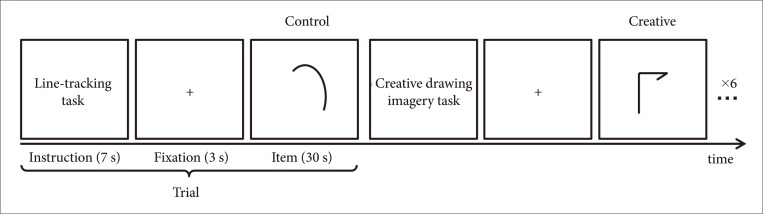
Fig. 2
Brain regions revealing greater activation during creative drawing imagery compared to line tracking condition. Group analysis revealed brain activation for creative drawing imagery condition than line tracking condition (creative>control) (p<0.001, uncorrected for multiple comparisons; cluster size≥50). The threshold activations were revealed on 3D brain images by projecting results on to the surface of the brain. 3D: 3-dimensional.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download