Abstract
Background and Purpose
Nicergoline is an ergoline derivative that is used to treat cognitive deficits in cerebrovascular disease and various forms of dementia. Although therapeutic effects of nicergoline have been established, little is known about its effects on cerebral perfusion in Alzheimer's disease (AD). The aim of this study was to examine the role of nicergoline in regional cerebral blood flow (rCBF) of AD patients using technetium-99m hexa-methyl-propylene-amine-oxime single photon emission computed tomography (SPECT).
Methods
Sixteen patients with early AD underwent a comprehensive clinical assessment including cognitive testing and SPECT scans before and after nicergoline treatment. Nicergoline (30 mg twice daily) was administered for an average duration of 1.5 years. Clinical and cognitive functioning was assessed using the Mini-Mental State Examination, Clinical Dementia Rating (CDR), CDR-Sum of Boxes, Global Deterioration Scale, Barthel Activities of Daily Living Index, Instrumental Activities of Daily Living, and Geriatric Depression Scale.
Results
Nicergoline treatment induced changes in the severity of dementia, cognitive function, activities of daily living, and depressive symptoms, which were not statistically significant. During the follow-up, the patients showed significant increases in their relative rCBF in the superior frontal gyrus, precentral gyrus, and postcentral gyrus.
Alzheimer's disease (AD) is the most common form of dementia and is clinically characterized by global deficits in cognition.1 Despite differences in etiology, AD and vascular dementia often coexist and have overlapping risk factors and pathologies.2 In addition to old age, the risk factors for AD include hypertension, peripheral arterial disease, cardiovascular diseases, diabetes, and smoking, and the mechanisms underlying AD appear to be closely associated with vascular factors.3
Nicergoline is an ergoline derivative that is used for the treatment of dementia and other age-related cognitive deficits.4 While nicergoline was initially developed as a vasodilator and mainly prescribed for cerebrovascular diseases, it has a wider spectrum of pharmacological and clinical properties leading to its use in various forms of dementia including AD. Several randomized controlled trials have investigated the therapeutic efficacy of nicergoline in patients with dementia and demonstrated that nicergoline treatment improved or prevented the deterioration of cognitive symptoms in AD patients,567 as well as in patients with senile dementia, vascular, or mixed type.891011
Nicergoline plays a role in the molecular and cellular pathophysiology of dementia. In in vitro and animal studies, nicergoline has been reported to act as an α1-adrenoceptor antagonist, resulting in vasodilation and blood flow increase,12 cholinergic neurotransmission,131415 enhanced noradrenaline and dopamine turnover,16 cerebral metabolic activity,1718 and neuroprotection.192021 Moreover, nicergoline has been shown to mediate neuronal signal transduction by modulating phosphoinositide pathway, protein kinase C (PKC) translocation, and PKC-mediated α-secretase processing of amyloid precursor protein implicated in the pathophysiology of AD.222324
While the clinical effects and potential mechanisms of nicergoline on AD have been studied, its effects on the brains of AD patients remain unclear. A single study used electroencephalography to measure neural activities correlated with nicergoline treatment in senile dementia of Alzheimer type and multi-infarct dementia.11 The aim of this study was to elucidate the effects of nicergoline on regional cerebral blood flow (rCBF) in early AD patients using single photon emission computed tomography (SPECT).
Sixteen patients with AD were recruited at Incheon St. Mary's Hospital (Incheon, Korea). The diagnosis of AD was made according to the Diagnostic and Statistical Manual of Mental Disorders-IV criteria,25 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria.26 Patients with a history of head trauma, epilepsy, stroke, mixed or vascular dementia, radiological findings on magnetic resonance imaging (MRI), or other neurological or psychiatric disorders were excluded from the study.
The study was approved by the Institutional Review Board of the Incheon St. Mary's Hospital. Written informed consent was obtained from all participants.
Patients received oral nicergoline at a dose of 30 mg twice daily for 1.5 years on average. All patients were also undergoing treatment with acetylcholinesterase inhibitors (AChEI) for AD at the time of the study. Safety assessments including adverse events, physical examinations, monitoring of vital signs, electrocardiography, and laboratory tests were performed.
All patients underwent a comprehensive clinical assessment including a detailed medical history and neurological examination, by board-certified neurologists. Global cognitive functioning was evaluated with the Mini-Mental State Examination (MMSE).27 Assessments for dementia severity included the Clinical Dementia Rating (CDR),28 CDR-Sum of Boxes (CDR-SB), and Global Deterioration Scale (GDS).29 The Barthel Activities of Daily Living Index (Barthel ADL Index)30 and Instrumental Activities of Daily Living (IADL)31 were used to evaluate functional status. The Geriatric Depression Scale (GDS-Depression) was used to assess depressive symptoms.32
SPECT images were acquired with a dual-head gamma camera (Discovery NM640, GE Healthcare, Milwaukee, WI, USA) at the baseline and follow-up visits. Participants were scanned approximately 40 min after a bolus intravenous injection of 1110 MBq of technetium-99m hexamethylpropylene amine oxime. All images were corrected for attenuation and reconstructed in a 128×128 matrix with a voxel size of 3.9×3.9×3.9 mm using filtered back projection.
Data were analyzed using Statistical Parametric Mapping (SPM) 12 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). All SPECT images were registered and spatially normalized to the SPM SPECT template (Montreal Neurological Institute, McGill University, Montreal, Canada) using a 12-parameter affine transformation and nonlinear warping with 25-mm cutoff and 16 iterations. Images were then re-sliced with a voxel size of 2×2×2 mm and smoothed with a 16-mm full-width half-maximum Gaussian kernel. After spatial normalization, a voxel-based intensity of the images was normalized to the mean value of the cerebellum using the Automated Anatomical Labeling atlas.333435
A paired t-test was used to examine changes in regional perfusion in the follow-up scans compared with the baseline on a voxel-by-voxel basis. Regions with the voxel subsets exceeding a threshold of p<0.001 and a cluster size of 100 or more contiguous voxels were reported as significant.
The Shapiro-Wilk test was used to determine the normality of distribution for each variable. Changes in continuous variables between the baseline and follow-up visits were performed with a paired t-test or Wilcoxon signed rank sum test. A two-tailed p value of less than 0.05 was regarded as statistically significant. All analyses were conducted with Stata 13 (Stata Corp., College Station, TX, USA).
Demographic and clinical characteristics of the participants are listed in Table 1. Sixteen patients (6 males and 10 females) with early AD were included in the study. The mean age at the baseline was 77.0±6.4 years. The mean duration between baseline and follow-up assessments was 1.5±0.5 years. The CDR scores remained unchanged during the study period. Moreover, changes in MMSE (t=−0.11, p=0.91), CDR-SB (t=−1.84, p=0.09), GDS (z=−1.00, p=0.32), IADL-C (t=−0.26, p=0.80), IADL-P (t=−1.16, p=0.26), Barthel ADL Index (z=1.84, p=0.07), and GDS-Depression (z=−0.57, p=0.57) scores from baseline to follow-up were not significant.
The results of image analysis showed significant increases in relative rCBF of the left superior frontal gyrus (t=4.90, p<0.001, cluster size=465 voxels), left postcentral gyrus (t=5.16, p<0.001, cluster size=112 voxels), and the left precentral gyrus (t=4.56, p<0.001, cluster size=105 voxels) at follow-up compared with baseline (Fig. 1 and Table 2). There were no significant decreases in relative rCBF.
In the present study, we investigated the effects of nicergoline on cerebral perfusion in early AD patients. We found that nicergoline treatment increased the relative rCBF in the left superior frontal gyrus, left postcentral gyrus, and left precentral gyrus. No significant differences in clinical measures were found at follow-up compared with baseline. Previous longitudinal studies investigating AD patients demonstrated a global reduction in rCBF36 and in cognitive and neuropsychiatric performance over a one-year period.3738 Deterioration in cerebral perfusion and cognitive performance represent the natural course of the disease. Our results suggest that nicergoline may have beneficial effects on AD by interfering with the degenerative processes.
Apart from the medial temporal lobe, which has been widely implicated in the pathophysiology of early AD,363940 previous neuroimaging studies of normal aging, mild cognitive impairment (MCI), and AD also found structural and functional deficits in the frontal and parietal regions. Using MRI, cross-sectional studies of normal aging have reported a non-linear and regional atrophy within the brain, and the prefrontal cortex declined more rapidly than the other brain regions.414243 A longitudinal study showed that gray matter atrophy was more prominent in the frontal and parietal cortices than temporal and occipital cortices in healthy older adults.44 In functional imaging studies using positron emission tomography and SPECT, studies have demonstrated a reduction in glucose metabolism and blood flow in the frontal and parietal regions in patients who progressed from MCI to AD.4546 Furthermore, regional decreases in cerebral metabolism and perfusion in the frontal and temporo-parietal regions were found in the early stages of AD.47484950
Our findings suggest that nicergoline treatment improved the brain perfusion in the frontal and parietal regions of early AD patients. In particular, the largest cluster was found in the superior frontal gyrus, which is associated with higher cognitive functions such as working memory.51 Moreover, our study found increased cerebral perfusion in the precentral and postcentral gyri, which correspond to the primary motor and sensory cortices. While sensory and motor dysfunctions in AD have received relatively little attention in the field of AD research, recent evidence suggests that sensory and motor changes may precede the cognitive symptoms associated with AD pathogenesis.52 Taken together, it is possible that increased perfusion in the frontal and parietal regions may delay or prevent progressive deterioration of cognitive functions in AD.
Limitations of the study include the relatively small sample size and the lack of a control group. Moreover, cognitive evaluation with a more comprehensive neuropsychological battery may have facilitated the detection of subtle changes in cognitive function during the study period. Finally, it is possible that the rCBF changes observed in our study may have been affected by AChEI. However, previous SPECT studies involving AD patients treated with long-term AChEI therapy showed no increases in rCBF535455 or the effects were mainly localized to the frontal lobe.5657
In conclusion, this was the first SPECT study, to our knowledge, to examine cerebral perfusion changes associated with nicergoline treatment in early AD patients. Nicergoline treatment resulted in a favorable outcome involving cerebral perfusion during early AD. Moreover, the clinical symptoms of AD remained stable without deterioration during the study period. Further studies involving larger sample sizes and placebo-controlled design are required to confirm, and elucidate the treatment effects and the underlying neural mechanisms of nicergoline in AD.
Figures and Tables
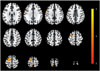 | Fig. 1Brain areas showing significantly increased regional cerebral blood flow in nicergoline-treated Alzheimer's disease patients compared with baseline. Images are shown in neurological convention. Color bar represents the voxel-level t-values. |
Table 1
Demographic and clinical characteristics of the participants

*Paired t-test or Wilcoxon signed rank sum test.
ADL: Activities of Daily Living, CDR: Clinical Dementia Rating, CDR-SB: Clinical Dementia Rating-Sum of Boxes, GDS: Global Deterioration Scale, GDS-Depression: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living-Current Performance (C) and Potential Performance (P), MMSE: Mini-Mental State Examination, SD: standard deviation.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2017R1C1B2011802).
References
1. Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: current status and new perspectives. Lancet Neurol. 2003; 2:539–547.

2. Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004; 35:11 Suppl 1. 2620–2622.

3. Skoog I, Kalaria RN, Breteler MM. Vascular factors and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999; 13:Suppl 3. S106–S114.

4. Winblad B, Carfagna N, Bonura L, Rossini BM, Wong EH, Battaglia A. Nicergoline in dementia: a review of its pharmacological properties and therapeutic potential. CNS Drugs. 2000; 14:267–287.
5. Bracco L, Bonura ML, Battaglia A. Six-month, multicentre, double-blind trial of nicergoline in the treatment of mild to moderate AD and its 12-month follow-up [abstract]. Neurosci Lett. 1999; 552:18.
6. Crook TH. Nicergoline in the treatment of probable Alzheimer's disease. Preliminary results of a double-blind, randomized, placebo-controlled study [abstract]. J Neurol Sci. 1997; 150:S18.
7. Winblad B, Bonura ML, Rossini BM, Battaglia A. Nicergoline in the treatment of mild- to- moderate Alzheimer's disease. Clin Drug Investig. 2001; 21:621–632.
8. Arrigo A, Moglia A, Borsotti L. A double-blind, placebo-controlled, crossover trial with nicergoline in patients with senile dementia [abstract]. Int J Clin Pharmacol Res. 1982; 2:4 Suppl 1. 33–41.
9. Battaglia A, Bruni G, Ardia A, Sacchetti G. Nicergoline in mild to moderate dementia. A multicenter, double-blind, placebo-controlled study. J Am Geriatr Soc. 1989; 37:295–302.

10. Nappi G, Bono G, Merlo P, Borromei A, Caltagirone C, Lomeo C, et al. Long-term nicergoline treatment of mild to moderate senile dementia: results of a multicentre, double-blind, placebo-controlled study. Clin Drug Investig. 1997; 13:308–316.
11. Saletu B, Paulus E, Linzmayer L, Anderer P, Semlitsch HV, Grünberger J, et al. Nicergoline in senile dementia of Alzheimer type and multi-infarct dementia: a double-blind, placebo-controlled, clinical and EEG/ERP mapping study. Psychopharmacology (Berl). 1995; 117:385–395.

12. Winblad B, Fioravanti M, Dolezal T, Logina I, Milanov IG, Popescu DC, et al. Therapeutic use of nicergoline. Clin Drug Investig. 2008; 28:533–552.

13. Carfagna N, Di Clemente A, Cavanus S, Damiani D, Gerna M, Salmoiraghi P, et al. Modulation of hippocampal ACh release by chronic nicergoline treatment in freely moving young and aged rats. Neurosci Lett. 1995; 197:195–198.

14. McArthur RA, Carfagna N, Banfi L, Cavanus S, Cervini MA, Fariello R, et al. Effects of nicergoline on age-related decrements in radial maze performance and acetylcholine levels. Brain Res Bull. 1997; 43:305–311.

15. Ogawa N, Asanuma M, Hirata H, Kondo Y, Kawada Y, Mori A. Cholinergic deficits in aged rat brain are corrected with nicergoline. Arch Gerontol Geriatr. 1993; 16:103–110.

16. Moretti A, Carfagna N, Caccia C, Carpentieri M. Effect of ergolines on neurotransmitter systems in the rat brain. Arch Int Pharmacodyn Ther. 1988; 294:33–45.
17. Le Poncin-Lafitte M, Grosdemouge C, Duterte D, Rapin JR. Simultaneous study of haemodynamic, metabolic and behavioural sequelae in a model of cerebral ischaemia in aged rats: effects of nicergoline. Gerontology. 1984; 30:109–119.

18. Miccheli A, Puccetti C, Capuani G, Di Cocco ME, Giardino L, Calzà L, et al. [1-13C]Glucose entry in neuronal and astrocytic intermediary metabolism of aged rats. A study of the effects of nicergoline treatment by 13C NMR spectroscopy. Brain Res. 2003; 966:116–125.

19. Caraci F, Chisari M, Frasca G, Canonico PL, Battaglia A, Calafiore M, et al. Nicergoline, a drug used for age-dependent cognitive impairment, protects cultured neurons against beta-amyloid toxicity. Brain Res. 2005; 1047:30–37.

20. Mizuno T, Kuno R, Nitta A, Nabeshima T, Zhang G, Kawanokuchi J, et al. Protective effects of nicergoline against neuronal cell death induced by activated microglia and astrocytes. Brain Res. 2005; 1066:78–85.

21. Sortino MA, Battaglia A, Pamparana F, Carfagna N, Post C, Canonico PL. Neuroprotective effects of nicergoline in immortalized neurons. Eur J Pharmacol. 1999; 368:285–290.

22. Caputi A, Di Luca M, Pastorino L, Colciaghi F, Carfagna N, Wong E, et al. Nicergoline and its metabolite induce translocation of PKC isoforms in selective rat brain areas. Neurosci Res Commun. 1998; 23:159–167.

23. Carfagna N, Cavanus S, Damiani D, Salmoiraghi P, Fariello R, Post C. Modulation of phosphoinositide turnover by chronic nicergoline in rat brain. Neurosci Lett. 1996; 209:189–192.

24. Cedazo-Minguez A, Bonecchi L, Winblad B, Post C, Wong EH, Cowburn RF, et al. Nicergoline stimulates protein kinase C mediated alpha-secretase processing of the amyloid precursor protein in cultured human neuroblastoma SH-SY5Y cells. Neurochem Int. 1999; 35:307–315.

25. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). Washington, DC: American Psychiatric Association;2000.
26. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.

27. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.
28. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39:1159–1165.
29. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean version of Global Deterioration Scale. J Korean Neurol Assoc. 2002; 20:612–617.
30. Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988; 10:64–67.

31. Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH, et al. The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL). J Korean Neurol Assoc. 2002; 20:8–14.
32. Jung IK, Kwak DI, Shin DK, Lee MS, Lee HS, Kim JY. A reliability and validity study of geriatric depression scale. J Korean Neuropsychiatr Assoc. 1997; 36:103–112.
33. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002; 15:273–289.

34. Pickut BA, Dierckx RA, Dobbeleir A, Audenaert K, Van Laere K, Vervaet A, et al. Validation of the cerebellum as a reference region for SPECT quantification in patients suffering from dementia of the Alzheimer type. Psychiatry Res. 1999; 90:103–112.

35. Soonawala D, Amin T, Ebmeier KP, Steele JD, Dougall NJ, Best J, et al. Statistical parametric mapping of (99m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer's disease: normalizing to cerebellar tracer uptake. Neuroimage. 2002; 17:1193–1202.

36. Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J Nucl Med. 2000; 41:1155–1162.
37. Benoit M, Robert PH, Staccini P, Brocker P, Guerin O, Lechowski L, et al. One-year longitudinal evaluation of neuropsychiatric symptoms in Alzheimer's disease. The REAL.FR Study. J Nutr Health Aging. 2005; 9:95–99.
38. Burns A, Jacoby R, Levy R. Progression of cognitive impairment in Alzheimer's disease. J Am Geriatr Soc. 1991; 39:39–45.

39. Masdeu JC, Zubieta JL, Arbizu J. Neuroimaging as a marker of the onset and progression of Alzheimer's disease. J Neurol Sci. 2005; 236:55–64.

40. Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002; 99:4703–4707.

41. Coffey CE, Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, et al. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992; 42(3 Pt 1):527–536.

42. Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet. 2004; 363:392–394.

43. Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997; 7:268–282.

44. Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003; 23:3295–3301.

45. Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003; 30:1104–1113.

46. Encinas M, De Juan R, Marcos A, Gil P, Barabash A, Fernández C, et al. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2003; 30:1473–1480.

47. Chase TN, Foster NL, Fedio P, Brooks R, Mansi L, Di Chiro G. Regional cortical dysfunction in Alzheimer's disease as determined by positron emission tomography. Ann Neurol. 1984; 15:Suppl. S170–S174.

48. Friedland RP, Budinger TF, Koss E, Ober BA. Alzheimer's disease: anterior-posterior and lateral hemispheric alterations in cortical glucose utilization. Neurosci Lett. 1985; 53:235–240.

49. Metter EJ, Riege WH, Kameyama M, Kuhl DE, Phelps ME. Cerebral metabolic relationships for selected brain regions in Alzheimer's, Huntington's, and Parkinson's diseases. J Cereb Blood Flow Metab. 1984; 4:500–506.

50. Perani D, Di Piero V, Vallar G, Cappa S, Messa C, Bottini G, et al. Technetium-99m HM-PAO-SPECT study of regional cerebral perfusion in early Alzheimer's disease. J Nucl Med. 1988; 29:1507–1514.
51. du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006; 129(Pt 12):3315–3328.

52. Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015; 11:70–98.

53. Kimura N, Kumamoto T, Masuda T, Hanaoka T, Okazaki T, Arakawa R. Evaluation of the regional cerebral blood flow changes during long-term donepezil therapy in patients with Alzheimer's disease using 3DSRT. J Neuroimaging. 2012; 22:299–304.

54. Nobili F, Koulibaly M, Vitali P, Migneco O, Mariani G, Ebmeier K, et al. Brain perfusion follow-up in Alzheimer's patients during treatment with acetylcholinesterase inhibitors. J Nucl Med. 2002; 43:983–990.
55. Ushuijima Y, Okuyama C, Mori S, Kubota T, Nakai T, Nishimura T. Regional cerebral blood flow in Alzheimer's disease: comparison between short and long-term donepezil therapy. Ann Nucl Med. 2006; 20:425–429.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download