1. Mesulam MM. Primary progressive aphasia--a language-based dementia. N Engl J Med. 2003; 349:1535–1542.

2. Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004; 55:335–346.

3. Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006; 129:1385–1398.

4. Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004; 56:399–406.

5. Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005; 128:1996–2005.

6. Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006; 59:952–962.

7. Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006; 59:156–165.

8. Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013; 81:1832–1839.

9. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010; 6:88–97.

10. Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015; 14:114–124.

11. Harada R, Okamura N, Furumoto S, Furukawa K, Ishiki A, Tomita N, et al. 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J Nucl Med. 2016; 57:208–214.

12. Harada R, Ishiki A, Kai H, Sato N, Furukawa K, Furumoto S, et al. Correlations of 18F-THK5351 PET with postmortem burden of tau and astrogliosis in Alzheimer disease. J Nucl Med. 2018; 59:671–674.

13. Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, et al. Monoamine oxidase B inhibitor, selegiline, reduces
18F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017; 9:25.

14. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011; 76:1006–1014.

15. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984; 34:939–944.

16. Kang Y, Jahng S, Na DL. Seoul Neuropsychological Screening Battery. 2nd ed. Seoul: Human Brain Research & Consulting Co.;2012.
17. Borroni B, Agosti C, Premi E, Cerini C, Cosseddu M, Paghera B, et al. The FTLD-modified Clinical Dementia Rating scale is a reliable tool for defining disease severity in frontotemporal lobar degeneration: evidence from a brain SPECT study. Eur J Neurol. 2010; 17:703–707.

18. Kim EJ, Park KW, Lee JH, Choi S, Jeong JH, Yoon SJ, et al. Clinical and neuropsychological characteristics of a nationwide hospital-based registry of frontotemporal dementia patients in Korea: a CREDOS-FTD study. Dement Geriatr Cogn Dis Extra. 2014; 4:242–251.

19. Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008; 131:2957–2968.

20. Lee JH, Kim SH, Kim GH, Seo SW, Park HK, Oh SJ, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011; 77:18–25.

21. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul neuropsychological screening battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076.

22. Kim H, Na DL. Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol. 2004; 26:1011–1020.

23. Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014; 55:1623–1628.

24. Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998; 39:904–911.
25. Rousset OG, Collins DL, Rahmim A, Wong DF. Design and implementation of an automated partial volume correction in PET: application to dopamine receptor quantification in the normal human striatum. J Nucl Med. 2008; 49:1097–1106.

26. Seelaar H, Kamphorst W, Rosso SM, Azmani A, Masdjedi R, de Koning I, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008; 71:1220–1226.

27. Mesulam M, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008; 63:709–719.

28. Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006; 66:41–48.

29. Kikuchi A, Okamura N, Hasegawa T, Harada R, Watanuki S, Funaki Y, et al.
In vivo visualization of tau deposits in corticobasal syndrome by 18F-THK5351 PET. Neurology. 2016; 87:2309–2316.

30. Vettermann F, Brendel M, Danek A, Levin J, Bartenstein P, Okamura N, et al. [18F] THK-5351 PET in patients with clinically diagnosed progressive supranuclear palsy. J Nucl Med. 2016; 57:457.
31. Ishiki A, Harada R, Okamura N, Tomita N, Rowe CC, Villemagne VL, et al. Tau imaging with [
18F]THK-5351 in progressive supranuclear palsy. Eur J Neurol. 2017; 24:130–136.

32. Gorno-Tempini ML, Ogar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006; 67:1849–1851.

33. Kremen SA, Mendez MF, Tsai PH, Teng E. Extrapyramidal signs in the primary progressive aphasias. Am J Alzheimers Dis Other Demen. 2011; 26:72–77.

34. Ferrari J, Pontello N, Martinez-Cuitiño M, Borovinsky G, Gleichgerrcht E, Torralva T, et al. Extrapyramidal signs across variants of primary progressive aphasias. Mov Disord. 2014; 29 Suppl 1:598.
35. Gulyás B, Pavlova E, Kása P, Gulya K, Bakota L, Várszegi S, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. 2011; 58:60–68.


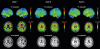
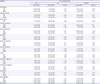
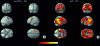




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download