Abstract
Background:
For creatinine measurement, the enzymatic method is known to be more accurate than the Jaffe method; however, the latter is still widely used. We evaluated the performance of the CRE2 reagent (Siemens Healthcare Diagnostics Inc., USA), which uses a modified Jaffe method.
Methods:
Three quality control standards were used for precision evaluations of CRE2 on Dimension VISTA 500 instrument (Siemens). Moreover, the linearity and carryover characteristics were assessed. Sixty-eight creatinine results obtained using the CRE2 and ECREA (enzymatic) reagents (Siemens) were compared with those obtained using the L-CRE (enzymatic) reagent (Shinyang Diagnostics, Korea). The accuracy of CRE2, ECREA, and L-CRE was evaluated using a standard reference material.
Results:
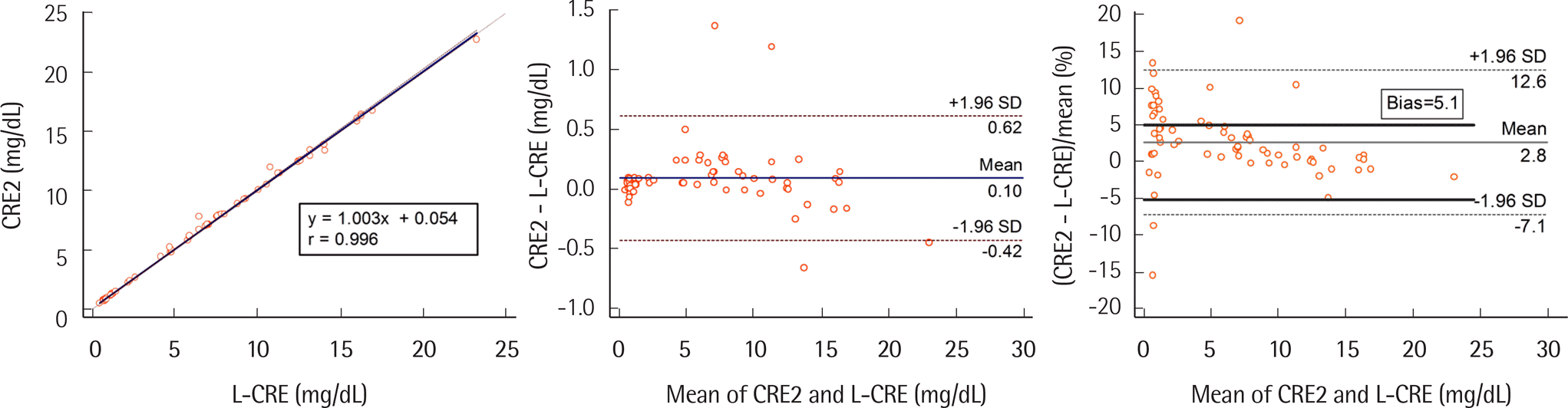
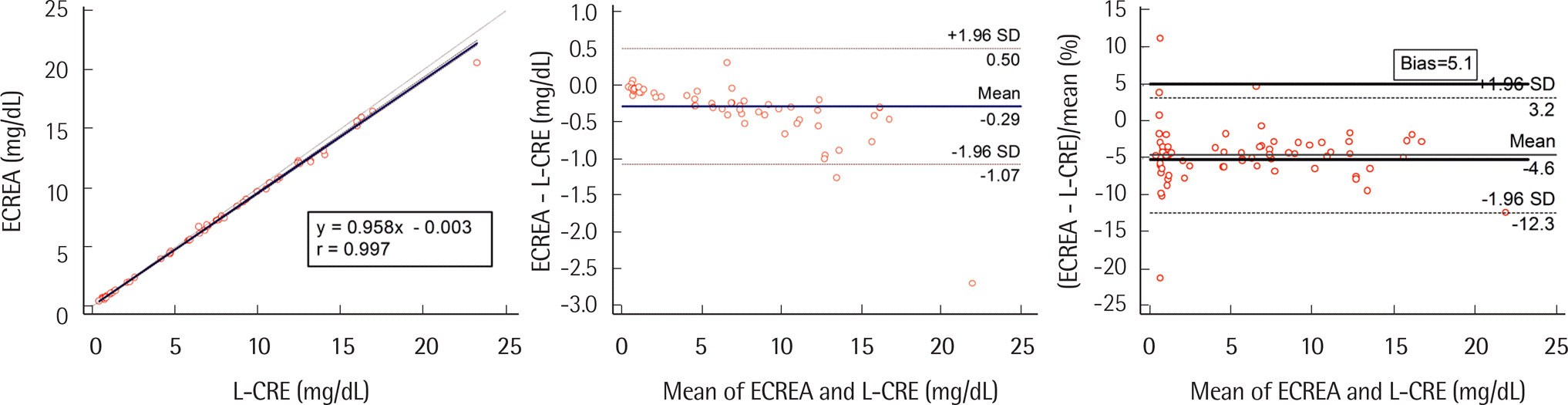
The CV of within-run (0.7–2.4%), between-run (0.4–1.7%), between-day precision (0.7–0.9%) for three standards, and total CV for medium (1.6%) and high levels (1.3%) satisfied the analytical goal. The linearity for CRE2 was excellent (R2=0.999). Comparisons of CRE2 and ECREA to L-CRE were well correlated (r=0.996 and 0.997, respectively). In comparison with L-CRE, 5 CRE2 results and 15 ECREA results exceeded minimum bias goal (5.1%) in samples with creatinine levels of >1 mg/dL. The carryover rate was -0.04%. In terms of accuracy, the percent bias values of CRE2, ECREA, and L-CRE were 7.4, -6.4, and -3.4, respectively, for low level; and 3.9, -1.5, and 0.7, respectively, for high level.
Go to : 
REFERENCES
1.Chung HJ., Chun SI., Min WK. Creatinine determination with minimized interference. J Lab Med Qual Assur. 2008. 30:229–31.
2.Burtis CA., Ashwood ER, et al. eds. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed.St. Louis, MO: Elsevier Inc.;2006. p. 797–801.
3.O'Leary N., Pembroke A., Duggan PF. A simplifed procedure for eliminating the negative interference of bilirubin in the Jaffe reaction for creatinine. Clin Chem. 1992. 38:1749–51.
4.Myers GL., Miller WG., Coresh J., Fleming J., Greenberg N., Greene T, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006. 52:5–18.

5.Siemens Dimension Vista® system Creatinine (CRE2) Flex® reagent cartridge. https://www.accessdata.fda.gov/cdrh_docs/pdf13/K133728.pdf.
6.Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline. 2nd ed.EP09-A2. Wayne, PA: Clinical and Laboratory Standard Institute;2002.
7.Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures; approved guideline. 3rd ed.EP05-A3. Wayne, PA: Clinical and Laboratory Standard Institute;2014.
8.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures; a statistical approach; approved guideline. EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute. 2003.
9.Clinical and Laboratory Standards Institute. Preliminary evaluation of quantitative clinical laboratory measurement procedures; approved guideline. 3rd ed.EP10-A3.Wayne, PA: Clinical and Laboratory Standards Institute;2006.
10.Robert L. National Institute of Standards and Technology certifcate of analysis, standard reference material. 665:2014. 1–3.
11.Nah H., Lee SG., Lee KS., Won JH., Kim HO., Kim JH. Evaluation of bilirubin interference and accuracy of six creatinine assays compared with isotope dilution-liquid chromatography mass spectrometry. Clin Bio-chem. 2016. 49:274–81.

12.Ricos C., Alvarez V., Cava F., Garcia-Lario JV., Hernandez A., Jimenez CV, et al. Desirable specifcations for total error, imprecision, and bias, derived from intra- and inter-individual biological variation. https://www.westgard.com/biodatabase1.htm. (updated on 2014).
13.Burtis CA., Ashwood ER, et al. eds. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed.St. Louis, Mo: Elsevier Inc;2006. p. 196–8.
14.Hermida FJ., Lorenzo MJ., Pérez A., Fernández M., Sagastagoia O., Maga-dán C. Comparison between ADVIA Chemistry systems Enzymatic Creatinine_2 method and ADVIA Chemistry systems Creatinine method (kinetic Jaffe method) for determining creatinine. Scand J Clin Lab Invest. 2014. 74:629–36.

15.Schmidt RL., Straseski JA., Raphael KL., Adams AH., Lehman CM. A risk assessment of the Jaffe vs enzymatic method for creatinine measurement in an outpatient population. PLoS ONE. 2015. 10:e0143205.

16.Panteghini M and IFCC Scientifc Division. Enzymatic assays for creatinine: time for action. Clin Chem Lab Med. 2008. 46:567–72.
Go to : 
Table 1.
Precision of CRE2 reagent for serum creatinine measurement using Dimension VISTA 500 instrument
| Level | Mean |
Within-run |
Between-run |
Between-day |
Total |
Desirable precision criteria (%)∗ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SD | CV (%) | SD | CV (%) | SD | CV (%) | SD | CV (%) | |||
| Low (0.538–0.801 mg/dL) | 0.629 | 0.015 | 2.4 | 0.011 | 1.7 | 0.006 | 0.9 | 0.020 | 3.1 | 2.98 |
| Medium (1.67–2.17 mg/dL) | 1.889 | 0.019 | 1.0 | 0.019 | 1.0 | 0.013 | 0.7 | 0.003 | 1.6 | |
| High (7.38–9.1 mg/dL) | 8.210 | 0.060 | 0.7 | 0.034 | 0.4 | 0.078 | 0.9 | 0.104 | 1.3 | |
Table 2.
Linearity of CRE2 reagent for serum creatinine measuremen using Dimension VISTA 500 instrument
| Test range (mg/dL) | Observed linear range (mg/dL) | Linear range (mg/dL) claimed by the manufacturer∗ | Slope | Intercept | R2 |
|---|---|---|---|---|---|
| 0.297–19.00 | 0.297–19.00 | 0.150–20.0 | 1.005 | 0.031 | 0.999 |
Table 3.
Comparison of CRE2 and ECREA reagents using Dimension VISTA 500 instrument with L-CRE reagent using Toshiba 2000FR Neo instru ment at the medical decision levels of serum creatinine using Passing-Bablok regression
| Reagent | Slope (95% CI) | Intercept (95% CI) | Decision level (mg/dL) | Expected value (mg/dL) | Expected bias (%) | Minimum bias goal (%)∗ |
|---|---|---|---|---|---|---|
| CRE2 | 1.003 (0.996–1.010) | 0.054 (0.036–0.085) | 0.6 | 0.656 | 9.3 | 5.1 |
| 1.6 | 1.659 | 3.7 | ||||
| 3.5 | 3.565 | 1.9 | ||||
| ECREA | 0.958 (0.951–0.966) | -0.003 (-0.025–0.012) | 0.6 | 0.572 | 4.7 | 5.1 |
| 1.6 | 1.530 | 4.4 | ||||
| 3.5 | 3.350 | 4.3 |
Table 4.
Accuracy of serum creatinine measurements using CRE2 and ECREA reagents on Dimension VISTA 500 instrument, and L-CRE reagent on Toshiba 2000FR Neo instrument
| Level [Certified con-centration value∗ (mg/dL)] | Low level (0.847±0.018) | High level (3.877±0.082) | ||||
|---|---|---|---|---|---|---|
| CRE2 | ECREA | L-CRE | CRE2 | ECREA | L-CRE | |
| Reagent | ||||||
| Mean (mg/dL) | 0.910 | 0.792 | 0.818 | 4.03 | 3.82 | 3.91 |
| Bias (mg/dL) | 0.063 | -0.055 | -0.029 | 0.15 | -0.06 | 0.03 |
| Percent bias (%) | 7.4 | -6.4 | -3.4 | 3.9 | -1.5 | 0.7 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download