Abstract
Purpose
To investigate the short-term efficacy and safety of ranibizumab in the routine clinical setting in patients with neovascular age-related macular degeneration and to analyze the associated factors for visual outcome.
Methods
This was a post-hoc analysis of a ranibizumab regulatory post-marketing surveillance study in which 4,136 patients were enrolled and followed for 12 weeks. Change in best-corrected visual acuity (BCVA), size of choroidal neovascularization, and the presence of hemorrhage and exudate were analyzed and the association between BCVA change and baseline characteristics were investigated. Data on ocular and systemic adverse events were collected.
Results
Mean BCVA improved significantly and mean BCVA change was the logarithm of the minimal angle of resolution 0.13 ± 0.01 (p < 0.001). A lower baseline BCVA and younger age were significant predictive factors for visual improvement or maintenance (≥0 lines). For greater visual acuity gain (≥3 lines), no treatment history, lower baseline BCVA, younger age, and classic-type choroidal neovascularization were significant predictive factors. No new safety signals were found.
Conclusions
In this study, conducted in real-world clinical practice with a large number of neovascular age-related macular degeneration patients, visual and anatomical outcomes improved significantly after three monthly ranibizumab treatments. Treatment-naive patients had a higher chance of greater visual gain (≥3 lines) than non-naive patients.
Age-related macular degeneration (AMD) is the most common cause of irreversible severe vision loss in the elderly in the developed world [1]. As the population ages, the number of people with AMD and the associated healthcare expenditures are likely to increase [2]. The estimated prevalence of AMD in the Korean population aged 40 years or older is 6.62%: 6.02% are in early AMD and 0.60% are in late AMD [3]. Of those in late AMD, 80% of cases are neovascular AMD (nAMD), which is characterized by choroidal neovascularization and is responsible for most of the severe vision loss from AMD. Lucentis (ranibizumab) was first approved as a treatment drug for nAMD in 2006 by the US Food and Drug Administration after two randomized controlled trials (RCTs) described superior visual outcomes in patients with nAMD who had received monthly ranibizumab injections compared with those treated by photodynamic therapy or observation [45].
Ranibizumab was approved in Korea by the Ministry of Food and Drug Safety on 27 July 2007 for the treatment of nAMD. Although several small studies have investigated the efficacy and safety of ranibizumab in Korean patients with nAMD since its approval [678], no large-scale data are available. This regulatory post-marketing surveillance (PMS) study was therefore conducted to evaluate the safety and efficacy of ranibizumab in Korean patients. Whereas most of the studies on Asian nAMD patients have taken place either in an RCT setting or retrospectively and have been limited by the small number of patients [678910], the strength of this PMS study lies in the enrollment of a large number of Korean patients from nearly all the centers specialized for retina care in South Korea. Furthermore, by enrolling patients who would not have met the strict inclusion criteria of an RCT, the PMS study ref lects the real-world efficacy and safety of ranibizumab for nAMD in South Korea. Through this study, we can also identify the demographic and clinical features of patients who visit hospitals and clinics for ranibizumab treatment.
Here, we report the short-term efficacy and safety of ranibizumab in a large number of patients with nAMD in the routine clinical setting and also analyze the associated factors that may influence visual outcome.
This was an observational study of nAMD patients who received intravitreal ranibizumab therapy in the routine clinical practice setting and were enrolled in the regulatory PMS study database. Brief ly, the PMS study was a multi-center, open-label, 12-week, prospective and observational study to evaluate the efficacy and safety of ranibizumab in routine clinical practice according to the Ministry of Food and Drug Safety of Korea. Investigators enrolled subjects consecutively from the first patient to receive ranibizumab until the planned number of subjects was reached and each subject was observed for at least 3 months from the first ranibizumab injection. Inclusion criteria for the PMS study were patients who had been prescribed ranibizumab for all approved indications at the time of treatment and who were available to be contacted by phone and be followed-up for details of ranibizumab administration. Exclusion criteria were the patients who had concurrent ocular and periocular infection, inflammation, and known hypersensitivity to ranibizumab. The first patient was enrolled on 25th January 2008 and the last patient completed the study on 6th March 2013. According to the Ministry of Food and Drug Safety guidelines, a PMS study should enroll ≥3,000 subjects. A total of 4,136 subjects were enrolled by 82 investigators from 58 clinical centers specialized in retinal disease treatment in Korea.
At every visit, best-corrected visual acuity (BCVA) was measured with Early Treatment Diabetic Retinopathy Study (ETDRS) charts or its equivalent such as Snellen visual acuity charts, and funduscopic evaluation was conducted. Fundus photography, fluorescein angiography (FA), and optical coherence tomography (OCT) were conducted at baseline and week 12. Adverse events were collected during the study period for safety analysis.
Institutional ethics approval for the PMS study was obtained from the institutional review board of each participating center and informed consent was obtained from each participant. The current post-hoc analysis was approved by the institutional review board of Seoul National University Bundang Hospital (B-1704-393-101). The research described adhered to the tenets of the Declaration of Helsinki.
In this post-hoc analysis of the PMS study, the inclusion criteria were nAMD patients who participated in the PMS study and who were treated with three monthly ranibizumab injections. Two study groups were selected from the total data set: a safety analysis set and efficacy analysis set (Fig. 1). Patients were excluded if they had enrollment errors, age less than 40 years, diagnosis of retinal diseases other than nAMD, fewer than three monthly ranibizumab injections, or a bilateral diagnosis of nAMD. Patients with bilateral nAMD were excluded due to lack of eye-specific data in their past medical history at baseline and on ranibizumab injection during the study period. After these exclusion criteria were applied, 2,938 nAMD patients were included in the safety analysis set. The efficacy analysis set included a total of 2,811 patients after excluding an additional 125 patients who lacked BCVA data and two patients whose initial BCVA was light perception.
In the efficacy analysis set, subjects who had past medical history of treatment for nAMD such as retinal laser treatment, intravitreal drug injection, verteporfin photodynamic therapy, and vitrectomy were defined as the non-naive group and subjects without any treatment history for nAMD were defined as the treatment-naive group.
BCVA values measured with ETDRS charts or its equivalent were converted to the logarithm of minimum angle resolution (logMAR) for analysis. The logMAR visual acuity was defined as 2.0 for counting fingers and 3.0 for hand motion [11]. The type (predominantly classic, minimally classic and occult without classic choroidal neovascularization [CNV]) at baseline and the change in the size of CNV after treatment were evaluated and reported by the treating physician using FA. The change in macular hemorrhage including subretinal and retinal hemorrhage and the change of exudation were also graded by the treating physician based on funduscopy, OCT, and/or FA at the final visit compared with baseline.
The primary outcome of the efficacy analysis was the change in mean BCVA at week 12 of all subjects and in the naive and non-naive groups at one month after three monthly ranibizumab injections. Secondary outcomes were the changes (improvement/no change/worsening) in anatomical outcomes including CNV size, macular hemorrhage, and the exudative status judged by the investigators. We also analyzed which demographic and ocular factors were associated with the short-term visual outcome after ranibizumab treatment. Finally, any adverse events occurring in the patients in the safety analysis set were summarized and reported.
Descriptive data are presented as mean ± standard deviation or number (%). Change of BCVA after ranibizumab treatment was calculated using the Wilcoxon signed rank test and described as mean (±standard error). The Wilcoxon rank sum test and the chi-square test were used for continuous and categorical variables, respectively, to compare baseline characteristics, efficacy, and safety variables between the naive and non-naive groups. We categorized eyes into four groups (≥3 lines gain, <3 lines gain or no change, <3 lines loss, ≥3 lines loss) by BCVA changes. Subjects were divided into two (<70 years, ≥70 years of age) or six (40–49, 50–59, 60–69, 70–79, 80–89, ≥90 years) age groups to investigate the association of age with BCVA change. Also, we divided the subjects into two (<20 / 100, ≥20 / 100) or four (<20 / 320, 20 / 320≤ to <20 / 100, 20 / 100≤ to <20 / 40, ≥20 / 40) groups according to their baseline BCVA. Comparisons of BCVA changes among the baseline BCVA groups, age groups, and groups of CNV types were performed using the Kruskal-Wallis test. To find factors associated with BCVA change, multiple linear regression analysis was then performed including factors that were significant in the univariable analysis. Logistic regression analysis was also performed to find the associated factors and their odds ratios with BCVA improvement of ≥3 lines and BCVA improvement or no change (≥0 lines), respectively. The patients were divided into two groups for age and baseline BCVA (model 1) or into six groups for age and four groups for baseline BCVA (model 2). All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA) and a p-value <0.05 was considered to indicate statistical significance.
The baseline characteristics of patients included in the efficacy analysis (n = 2,811) and safety analysis (n = 2,938) sets are described in Table 1 and 2. Among the patients in the efficacy analysis set, 2,167 patients were treatment naive and 644 patients were non-naive. The average age of patients in the efficacy analysis set was 69.4 ± 9.2 years and the majority were male (62.5%). The mean baseline BCVA of the total patients was logMAR 0.86 ± 0.57 (Snellen equivalent of 20 / 145). In this study, 701 (24.9%) patients with poor BCVA (less than 20 / 320) were included. There was no difference between the naive and non-naive groups in terms of gender, laterality of study eyes, and baseline BCVA. Conversely, the distribution of age groups and CNV types was different between the two groups: naive patients were slightly younger and had a higher proportion of classic CNV compared to non-naive patients (Table 1).
In the efficacy analysis set, the mean BCVA improved from logMAR 0.86 ± 0.57 to 0.73 ± 0.57 (Snellen visual acuity, from 20 / 145 to 20 / 107) after three monthly ranibizumab injections (p < 0.001) (Fig. 2A). The mean BCVA change was logMAR 0.13 ± 0.01, which is equivalent to a 6.5-letter gain (Table 3). BCVA significantly improved in both naive and non-naive groups, but the naive group showed statistically greater improvement in vision (logMAR −0.15 ± 0.01 [equivalent to a 7.5-letter gain]) than the non-naive group (logMAR −0.08 ± 0.01 [equivalent to a 4-letter gain], p < 0.001) (Fig. 2B). Eyes with occult CNV without a classic component improved the least compared with the other types of CNV (p < 0.001) (Fig. 2C).
In total, 28.1% of patients experienced ≥3 lines of BCVA gain, 52.2% gained <3 lines or no change, 10.8% lost <3 lines, and 8.9% lost ≥3 lines. The distribution of visual gain or loss was statistically significantly different between the naive and non-naive groups (p < 0.001) (Fig. 3). The most prominent difference between the groups is that 30.2% gained ≥3 lines in the naive group whereas 21.1% gained ≥3 lines in the non-naive group. The proportion of eyes that lost ≥3 lines at 3 months was less than 10% in both groups.
The size of CNV decreased in 68.9% of all patients (n = 2,811) and retinal or subretinal hemorrhage as well as exudate improved in more than 70% of patients after three monthly ranibizumab injections (Fig. 4A–4C). In all three anatomical parameters, a greater proportion of the naive group showed an improvement compared to the non-naive group (Table 3).
Univariable analysis showed treatment history, age, baseline BCVA, and CNV type were associated with BCVA change whereas gender and laterality (right vs. left eyes) were not associated. Treatment history was significantly associated with visual outcome and the treatment-naive group showed greater improvement in vision than the non-naive group (logMAR −0.15 vs. −0.08, 7.5 vs. 4 letters, p < 0.001). The BCVA changes were significantly greater in younger age groups (<70 years) compared to older age groups (≥70 years) (logMAR −0.15 vs. −0.11, 6.5 vs. 5.5 letters, p < 0.001 by Wilcoxon rank sum test) (Fig. 5A–5C) The BCVA change was significantly different among the six age groups and there was a trend for greater BCVA gain with decreasing age (p = 0.012 by Kruskal-Wallis test, r = 0.062, p = 0.001 by correlation analysis) (Fig. 5). Regarding the baseline BCVA, the lower baseline BCVA group (<20 / 100) showed greater visual improvement than the better baseline BCVA group (≥20 / 100) (logMAR −0.22 vs. −0.05, p < 0.001) and there was a trend for greater BCVA gain with worse baseline BCVA (p < 0.001 by Kruskal-Wallis test r = −0.331, p < 0.001 by correlation analysis) (Fig. 6A–6C).
The multiple linear regression analysis revealed that the history of no prior treatment, younger age, and worse baseline BCVA were significant predictive factors for greater BCVA improvement. BCVA change can be estimated by the equation: BCVA change (logMAR) = −0.2316 (p = 0.0002) + 0.0057 × age (yr, p < 0.0001) − 0.0616 × treatment history (p = 0.0002) − 0.2417 × baseline BCVA (logMAR, p < 0.0001) + 0.0091 × CNV types (p = 0.2389) (R2 = 0.1411, p < 0.0001).
Logistic regression analyses were conducted to reveal the odds ratios of baseline parameters for visual improvement or maintenance (BCVA gain ≥0 lines) and BCVA improvement ≥3 lines (Table 4, 5 and Fig. 7A–7D). Younger age and lower baseline BCVA showed a trend for higher odds ratio for greater BCVA improvement. However, no treatment history showed a significant odds ratio for ≥3 lines of BCVA gain whereas it was not significant for visual improvement or maintenance. Patients were divided into two age groups and two baseline BCVA groups in model 1 and they were further divided into six age groups and four baseline BCVA groups in model 2. The result was similar between models 1 and 2.
Ocular and systemic adverse events are summarized in Table 6. There were 37 (0.01%) adverse events and three of them were serious adverse events. Overall, no new safety signal was detected compared to the previous ranibizumab clinical trials [45]. There was no significant difference in the rate of ocular and systemic adverse events between the treatment-naive and non-naive groups.
Our study reports the short-term treatment outcomes of ranibizumab for nAMD in the real world and it encompassed the largest number of nAMD patients treated with ranibizumab ever studied in Korea. According to the National Claim Database of South Korea, 20,314 patients were treated with 71,574 ranibizumab injections from August 2009 to December 2012 (unpublished data). Therefore, our cohort of 2,938 nAMD patients treated with 7,814 ranibizumab injections represents approximately 15% of the total n AMD patients who were treated with ranibizumab in South Korea during the study period.
This observational study based on the PMS study revealed the short-term efficacy and the associated factors as well as the safety profile of ranibizumab in nAMD patients. Three monthly injections of ranibizumab resulted in a mean visual improvement of logMAR 0.13 (6.5 letters equivalent) and no serious adverse events occurred. No history of prior treatment, lower baseline BCVA, and younger age were associated with greater visual improvement. Predominantly or minimally classic CNV showed better visual gains than occult CNV in a univariable analysis, but not in a multivariable analysis.
The visual gain of 7.5 letters (logMAR 0.15) after three monthly injections of ranibizumab in our treatment-naive patient group was comparable to previous prospective RCTs including MARINA, ANCHOR, CATT, and VIEW [451213]. The visual gain at three months was not specifically reported in those trials, but the visual improvement plateaued after three monthly administrations and was maintained for up to one year. The visual gain at one year was 7.2 letters in MARINA, 11.3 letters in ANCHOR, 8.5 letters in CATT, and 9.3 letters in VIEW. Therefore, the efficacy of ranibizumab seen in the selected patient groups in the RCTs was well validated in the real-world clinical environment.
Patients with prior treatment history showed less BCVA gain than treatment-naive patients. The difference between groups was most prominent in the proportion of patients showing a visual gain of ≥3 lines (30.2% in naive group vs. 21.1% gained in non-naive group). This was also confirmed by logistic regression analysis and the naive group showed an odds ratio of 1.226 for visual improvement or no change and 1.672 for a BCVA gain ≥3 lines in model 1 (Table 4). This indicates that no treatment history was more associated with a higher level of visual improvement while it was less associated with modest visual improvement. Thus, we can expect visual improvement in both treatment-naive and non-naive patients, but greater visual improvements would be expected in the treatment-naive patients. In the non-naive group, 59.2% of patients were treated with an anti-vascular endothelial growth factor (VEGF) before enrollment in the PMS study, and this may have affected the slightly higher baseline BCVA in the non-naive group (naive vs. non-naive logMAR, 0.87 vs. 0.83, p = 0.082). Considering that t he final BCVA w as similar in t he t wo groups, the better baseline BCVA might have contributed to the lower BCVA gain in the non-naive group. Conversely, the worse visual outcome of the non-naive group might be related to the longer disease duration and greater loss of photoreceptors, which reduces the potential for visual improvement. It has been reported that baseline geographic atrophy is associated with worse visual outcome after ranibizumab treatment [1415]. Results from the CATT trial suggest that anti-VEGF treatment might accelerate the development of geographic atrophy [161718]. Most of our patients in the non-naive group were previously treated with bevacizumab. Thus, patients in the non-naive group might have a higher chance of having baseline geographic atrophy and photoreceptor loss than those in the naive group, which could have resulted in smaller visual improvement after treatment.
In our study, the lower baseline BCVA group (<20 / 100) showed greater visual improvement than the better baseline BCVA group (≥20 / 100) (logMAR −0.22 vs. −0.05, 11 vs. 2.5 letters). The lower baseline BCVA group (<20 / 100) were 4.6 times more likely to have ≥3 lines of visual gain compared with the higher BCVA group. In addition, there was a trend for greater BCVA gain with worse baseline BCVA (Fig. 6). The smallest visual gain in the best baseline visual acuity group (>20 / 40) and the greatest visual gain in the worst baseline visual acuity group (<20 / 320) can be explained by ceiling and floor effects. Patients in the least impaired baseline visual acuity group had the lowest potential to improve whereas patients who had poor baseline visual acuity had little possibility to have further loss of vision.
An important strength of our study is that it had no strict inclusion and exclusion criteria and, thus, it could reveal the treatment outcomes in real clinical practice and can provide answers to questions that well-designed randomized clinical trials cannot address. The most striking difference of this study from other clinical trials is that a substantial portion (24.9%) of patients had very poor visual acuity (less than 20 / 320) prior to ranibizumab treatment. Thus, the baseline BCVA of patients included in this study was relatively poor with logMAR visual acuity of 0.86 ± 0.57 (Snellen equivalent of 20 / 145). In most of the other clinical trials including the pivotal trials of ranibizumab [451213], patients with BCVA less than 20 / 320 were excluded. Therefore, there is insufficient evidence that ranibizumab can be beneficial for patients with a BCVA of less than 20 / 320. In routine clinical practice, nAMD patients with poor baseline visual acuity might be denied treatment by the physician. In our study, however, we found that the nAMD patients with BCVA <20 / 320 had significant improvement in vision after treatment and, notably, this group showed the greatest improvement in BCVA (Table 5 and Fig. 6). This result indicates that nAMD patients with poor vision should be encouraged to receive treatment if there is disease activity. In addition, our result should be considered when calculating the cost-benefit ratio of treatment for the poor vision nAMD patient group and when planning for the social and medical costs and insurance reimbursement guidelines.
Age-dependent efficacy of ranibizumab was also found in our study. The younger patients had greater potential for improving their vision than the older patients (Table 4, 5 and Fig. 5). Although this association was identified in many subgroup analysis reports [1415192021], the underlying reason has not been explained. It may be that the photoreceptors and retinal pigment epithelium cells in younger patients can be better repaired than in older patients, similar to other tissues in the human body [22]. The association of older age and better baseline visual acuity with worse visual gain is generally consistent with the pivotal trials and RCTs [1415192021]. In the CATT trial [1415], better baseline visual acuity, older age, and geographic atrophy were associated with better 1-year or 2-year visual gain. However, in the CATT trial, the visual gain was greatest in the baseline visual acuity group of 20 / 100 to 20 / 160, which was 11.9 letters at one year and 14.3 letters at two years. The group with baseline visual acuity of 20 / 200 to 20 / 320 showed the third and second greatest visual gain of 7.9 letters at one year and 10.5 letters at two years. This is slightly different from our result that the worst baseline BCVA group (<20 / 320) showed the greatest visual gain among all groups. In addition, in the CATT trial, predominantly or minimally classic CNV showed worse one-year visual gain, which is in contrast to our result. The discrepancy might be due to the difference in the study population and the study designs. In particular, the short-term measurement of visual outcome in our study might also have affected the association.
As this study encompassed most retinal centers in South Korea, it provides data on the general clinical features of nAMD patients in our country. The prior epidemiologic study using the Korean National Claim Database including 81,513 nAMD patients described very accurate prevalence, incidence, gender, and age distribution for nAMD [23]. However, the ophthalmic features and treatment outcomes could not be identified from the claims database. Our study of approximately 3,000 nAMD patients who were treated in the retina centers could reveal additional data including the mean visual acuity at diagnosis, CNV types, laterality of newly treated nAMD patients, and treatment efficacy in terms of functional and anatomical outcomes. In our study, the male-to-female ratio was 1.67, which was higher than that (1.03) in the Korean National Claim Database study [23]. Another study using Korean National Health and Nutritional Survey data reported a higher prevalence of late AMD in males than in females [3]. In other observational studies in different countries, the number of female patients was significantly higher than male patients [242526]. Although the exact reason behind the larger number of male patients is not known, it could be associated with a higher prevalence of nAMD in the Korean male population or it could be that male patients tend to visit specialized retina centers for treatment of nAMD whereas female patients visit smaller clinics for treatment. The choice of hospital or clinic might also be affected by the economic status of patients.
In our study, three monthly injections of ranibizumab showed a good safety profile. There were no cases of serious systemic adverse events such as stroke or myocardial infarction. The study period of three months might be too short to reveal the long-term safety of ranibizumab. But considering the possible combined morbidity and mortality due to the old age of nAMD patients and the lack of short-term adverse events from three monthly injections, it can be regarded that ranibizumab has minimal direct complications and it is tolerable at least up to three months. Other data from long-term clinical trials suggest that ranibizumab also has a good safety profile in the long-term and most adverse events were related to the procedure itself and to the old age of the patient population [451213].
There are similar observational studies that have investigated the real-world efficacy and safety of ranibizumab for nAMD. The COMPASS study was a multicenter, prospective, observational, 15-month study including a three-month loading phase using ranibizumab [25]. The efficacy population comprised 1,729 German nAMD patients, and the BCVA improved from 0.201 to 0.219 in decimal notation (Snellen equivalent: from 20 / 100 to 20 / 91) after three injections. Compared to our study of baseline BCVA of logMAR 0.86 (Snellen equivalent: 20 / 145), the patients in the COMPASS study had better baseline visual acuity, which might have resulted in less visual improvement. The incidence rate of adverse events in the COMPASS study was 5.6%, which is slightly higher than our study but lower than the CATT trial rate of 31.7% [27]. The low incidence of adverse events in our study might be due to the short follow-up period compared to other studies. As the authors concluded [25], there is also reduced reporting of adverse events or serious adverse events compared with the formal RCTs. In a report on the LUMINOUS study in which 4,444 nAMD patients were enrolled and followed-up for one year from four completed ranibizumab n AMD registries including German WAVE (Lucentis in Wet AMD: Evaluation of Visual Acuity and Quality of Life), HELIOS (Health Economics with Lucentis in Observational Settings) Netherlands, HELIOS Belgium and Swedish registry, patients gained +0.8 to 4.6 letters at one year with a mean number of 4.3 to 5.7 injections of ranibizumab [24]. The incidence of adverse events at one year was 1.64% for ocular adverse events, 0.43% for stroke and 0.11% for myocardial infarction. The LUMIERE study was a retrospective, observational study including 551 nAMD patients treated with ranibizumab [26]. After three monthly ranibizumab treatments, visual acuity improved by 6.7 ± 13.1 letters and at 12 months, the visual gain was 3.4 ± 15.8 letters. The visual gains in our study are comparable to these observational studies after three monthly treatments whereas they are smaller than the well-designed RCTs. This may be because of the inclusion of patients who might not be eligible for RCTs such as patients with prior treatment history, confounding ocular diseases, and without disease activity.
There are several limitations in this study of note. First, this was an observational study with no detailed inclusion criteria. The diagnosis of nAMD and the treatment decision were at the discretion of diverse retinal specialists. Thus, heterogeneous nAMD patients with wide spectrums of baseline clinical characteristics were included and these might have affected the outcome analysis. Second, the follow-up period was short at 12 weeks including three monthly ranibizumab injections. The long-term efficacy and associated factors as well as safety cannot be determined by our study alone. Third, additional potential prognostic data such as central retinal thickness and presence of intraretinal or subretinal fluid on OCT were not collected during the PMS study and were not included in this post-hoc analysis. Fourth, visual acuity worse than 20/320 such as counting fingers and hand motion might not have been exactly measured and the one-log difference between counting fingers (2.0) and hand motion (3.0) might be too large compared to visual acuities >20 / 320. These might have caused a large visual improvement in the lowest vision group. Lastly, our study subjects might not reflect the total nAMD patients in South Korea. During the study period, nAMD can be treated with either ranibizumab or off-label bevacizumab. Ranibizumab was reimbursed by the national healthcare but the price of the deductible was still similar to that of off-label bevacizumab and there was a reimbursement limitation in injection numbers of five times per patient. Therefore, not all the nAMD patients requiring anti-VEGF treatment would not have been treated with ranibizumab and some patients might have still been treated with bevacizumab during the study period. Thus, our study might not ref lect the clinical features of all nAMD patients in South Korea. However, the large number of n AMD patients treated w ith r anibizumab in our study a re a n indication of the general demographic a nd clinical features of nAMD patients in the real world.
In conclusion, the initial treatment of three monthly ranibizumab injections for nAMD showed good efficacy and safety in real world clinical practice that were comparable to RCTs. No prior treatment, younger age, and worse baseline visual acuity were associated with better visual outcomes. In particular, patients with poor baseline visual acuity benefited the most from ranibizumab treatment and naive patients experienced the greatest visual improvement of 3 or more lines.
Figures and Tables
Fig. 1
Flow charts of grouping subjects enrolled in the ranibizumab regulatory post-marketing surveillance study. CRF = case report form; DME = diabetic macular edema; RVO = retinal vein occlusion; CNV = choroidal neovascularization; CSCR = central serous chorioretinopathy; SRNVM = subretinal neovascular membrane; BCVA = best-corrected visual acuity.
Fig. 2
Changes of best-corrected visual acuity after three monthly ranibizumab injections. (A) Best-corrected visual acuity (BCVA, mean ± standard deviation) of 2,811 subjects in the efficacy analysis set improved at three months from baseline (Wilcoxon signed rank test, p < 0.001). (B) Eyes of treatment-naive group achieved greater BCVA (mean ± standard error) gain compared to those of non-naive group (Wilcoxon rank sum test, p < 0.001). (C) Changes of BCVA were different among the three different choroidal neovascularization (CNV) groups (Kruskal-Wallis test, p < 0.001). logMAR = logarithm of minimum angle resolution.

Fig. 3
Proportion of patients with visual change categorized as logarithm of the minimum angle of resolution ≤−0.3 (Early Treatment Diabetic Retinopathy Study 3 or more lines gain); 0 to −0.3 (<3 lines gain or no change); 0.3 to 0 (<3 lines loss); and 0.3 (3 or more lines loss) in total, treatment-naive, and non-naive groups. The distribution of visual gain or loss was statistically different between the naive and non-naive groups (chi-square test, p < 0.001). BCVA = best-corrected visual acuity.

Fig. 4
Anatomical changes including choroidal neovascularization (CNV) size (A), retinal or subretinal hemorrhage (B), and exudate (C) after three monthly injections of ranibizumab on the total patients (n = 2,811) in the efficacy analysis set.

Fig. 5
Comparison of best-corrected visual acuity (BCVA) changes according to the age groups. (A) BCVA (mean ± standard error) improved in both <70 and ≥70 years age groups significantly from baseline (Wilcoxon signed rank test, both p < 0.001), however, the change is greater in the younger age group (Wilcoxon rank sum test, p < 0.001). (B) BCVA improved in all age groups (Wilcoxon signed rank test, p < 0.05) except for the oldest age group (p = 0.074) and the change in BCVA was different among the six age groups (Kruskal-Wallis test, p < 0.001). (C) BCVA change was correlated with age (r = 0.062, p = 0.001). logMAR = logarithm of minimum angle resolution.

Fig. 6
Comparison of best-correct visual acuity (BCVA) changes according to the baseline visual acuity. (A) BCVA (mean ± standard error) improved in both <20 / 100 and ≥20 / 100 groups significantly from baseline (Wilcoxon signed rank test, both p < 0.001) and the change was greater in the worse baseline visual acuity group (Wilcoxon rank sum test, p < 0.001). (B,C) BCVA improved in three baseline BCVA groups (Wilcoxon signed rank test, p < 0.05) but not for the group with the best baseline BCVA ( p = 0.351). The changes in BCVA were different among the four baseline BCVA groups (Kruskal-Wallis test, p < 0.001) and correlated with baseline BCVA (r = 0.331, p < 0.001). logMAR = logarithm of minimum angle resolution.

Fig. 7
Graphs showing baseline predictive factors associated with (A,B) a visual gain or no change from baseline best-corrected visual acuity (BCVA) and those associated with (C,D) a gain of 3 or more lines from baseline BCVA. (A) Age less than 70 years and baseline BCVA worse than 20 / 100 were the significant predictors for BCVA gain or no change (p = 0.083 and p < 0.001, respectively) in model 1. (B) In model 2, age group 40 to 49 years (p = 0.049) and baseline BCVA group <20 / 320, 20 / 320 to 20 / 100, 20 / 100 to 20 / 40 were the significant predictors for BCVA gain or no change. (C) No treatment history (p < 0.001), age less than 70 years (p < 0.001), baseline BCVA worse than 20 / 100 (p < 0.001), choroidal neovascularization (CNV) with predominantly classic CNV (p = 0.002), and minimally classic CNV (p = 0.017) were associated with BCVA gain of 3 or more lines. (D) In model 2, no prior treatment history (p < 0.001), age of 40 to 49 years (p = 0.004), baseline BCVA of <20 / 320, 20 / 320 to 20 / 100, 20 / 100 to 20 / 40 (all p < 0.001), predominantly classic CNV (p = 0.005), and minimally classic CNV (p = 0.024) were the significant predictors for BCVA gain of three or more lines. *Versus occult without classic CNV.

Table 1
Baseline demographics and characteristics of subjects included in the efficacy analysis set (n = 2,811)
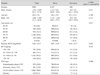
Table 2
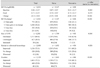
Baseline demographics a nd characteristics of subjects included in the safety analysis set (n = 2,938)

Table 4
Association of logMAR visual acuity change with prior-treatment history, age, baseline BCVA, and type of choroidal neovascularization (model 1)

Acknowledgements
The authors thank the investigators (Jae Kyoun Ahn, Gi Jung An, Suk H o B yeon, J u B yung Chae, Moo H wan Chang, Woo Hyok Chang, Ho Kyun Cho, Sung Won Cho, Young Jae Cho, Young Wook Cho, Gwang Ju Choi, Young Kwang Chu, Eun Jee Chung, Hum Chung, In Young Chung, Jang Won Heo, Beom Nun Hwang, K uhl Huh, Young Joon Jo, Woo Jin Jung, Jae Hoon Kang, Hyung Woo Kwak, Eui Yong Kweon, Oh Woong Kwon, Ha Kyoung K im, Hae Gon K im, Han Gyu K im, Hyoung Kyun Kim, Hyun Woong Kim, In Taek Kim, Jeong Yong Kim, June Gone Kim, Jung Yeul Kim, Kwang Soo Kim, Min Ho Kim, Si Dong Kim, Soon Hyun Kim, Sung Soo Kim, Tae Wan Kim, Young Gyun Kim, Yu Cheol Kim, Hyoung Jun Koh, Dong Heun Nam, Dong Won Lee, Eun Koo Lee, Ji Eun Lee, Jong Hyun Lee, Joo Yong Lee, Joo Eun Lee, Sang Joon Lee, Seong Jun Lee, Seung Jun Lee, Seungkyu Lee, Sung Chul Lee, Tae Gon Lee, Won Ki Lee, Sung Jin Na, Jong Hyun Oh, Jae Yoon Oh, Young-Hoon Ohn, Boo Sup Oum, In Won Park, Jong Seok Park, Jung Min Park, Kyu Hyung Park, Ho Ra, Young Jung Rho, Min Sagong, Seong Joo Shin, Joon Hong Sohn, Ji Hun Song, Won Kyung Song, Je Moon Woo, Jae Wook Yang, Jee Wook Yang, Yun Sik Yang, Yong Sung You, Hyeong Gon Yu, Seung Young Yu, Sun Im Yu, In Han Yun) for their contribution to ranibizumab regulatory PMS study and Hae Jin Kwon, M.S. in Biostatistics, LSK Global PS, for statistical support.
Notes
Conflict of Interest Se Joon Woo is a paid consultant of Samsung Bioepis Inc., Songdo, South Korea and is a co-founder of RetiMark, a bio-venture company in Seoul, South Korea. Ga Eun Cho is an employee of Novartis Korea, Seoul, South Korea. Novartis Korea sponsored the study and was involved in the study conception, design, protocol writing, study coordination, data analysis, data interpretation, manuscript writing, English editing and submission. Joon Hee Cho has nothing to declare.
References
1. Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004; 291:1900–1901.
2. Lee PP, Feldman ZW, Ostermann J, et al. Longitudinal prevalence of major eye diseases. Arch Ophthalmol. 2003; 121:1303–1310.


3. Park SJ, Lee JH, Woo SJ, et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–1765.

4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.


5. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.


6. Kim JH, Lee DW, Chang YS, et al. Twelve-month outcomes of treatment using ranibizumab or aflibercept for neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2016; 254:2101–2109.


7. Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013; 156:652–660.


8. Shin JY, Yu HG. Optical coherence tomography-based ranibizumab monotherapy for retinal angiomatous proliferation in Korean patients. Retina. 2014; 34:2359–2366.


9. Ogura Y, Terasaki H, Gomi F, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. Br J Ophthalmol. 2015; 99:92–97.


10. Lee FL, Kwon OW, Chung H, et al. Ranibizumab in South Korean and Taiwanese patients with age-related macular degeneration: primary outcome of the EXTEND III study. Acta Ophthalmol. 2012; 90:e406–e407.

11. Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997; 13:388–391.


12. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.



13. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.

14. Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013; 120:122–129.


15. Ying GS, Maguire MG, Daniel E, et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2015; 122:2523–2531.



16. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014; 121:150–161.

17. Grunwald JE, Pistilli M, Daniel E, et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017; 124:97–104.


18. Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015; 122:809–816.


19. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007; 114:246–252.


20. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007; 144:850–857.


21. Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011; 118:523–530.


22. Chen M, Rajapakse D, Fraczek M, et al. Retinal pigment epithelial cell multinucleation in the aging eye: a mechanism to repair damage and maintain homoeostasis. Aging Cell. 2016; 15:436–445.


23. Park SJ, Kwon KE, Choi NK, et al. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015; 122:2063–2070.

24. Holz FG, Bandello F, Gillies M, et al. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol. 2013; 97:1161–1167.



25. Wolf A, Kampik A. Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol. 2014; 252:647–655.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download