Abstract
Acinetobacter is an important opportunistic, multidrug resistant pathogen causing majority of nosocomial infections worldwide. The multidrug resistance is attributed by a plethora of efflux pumps and the overexpression of the same mediates export of antimicrobial agents. Quorum sensing (QS) is the cell-to-cell communication system in which bacteria produces specific signaling molecules which are transported out to the surrounding environment to communicate with other bacterial cells. It has been noticed that multidrug efflux pumps like resistance-nodulation-cell division (RND) efflux pumps play an important role in QS by exporting these signaling molecules. This review discusses various RND efflux pumps and the current understanding of the interrelationship of RND efflux pumps and QS in Acinetobacter spp. Studies demonstrate that RND efflux pumps could be considered as potential targets to block QS thereby reducing pathogenesis and antibiotic resistance. The known RND efflux pump-mediated quorum quenching strategies for Acinetobacter and other bacterial strains are discussed in detail. Finally, the prospective quorum quenching strategies targeting the transcriptional regulators of RND efflux pumps to inhibit multidrug efflux pumps are addressed.
Go to : 
REFERENCES
1). Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000; 406:775–81.

2). Aarestrup FM. Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic Clin Pharmacol Toxicol. 2005; 96:271–81.

4). Davin-Regli A, Bolla JM, James CE, Lavigne JP, Chevalier J, Garnotel E, et al. Membrane permeability and regulation of drug "influx and efflux" in enterobacterial pathogens. Curr Drug Targets. 2008; 9:750–9.
5). Blair JM, Richmond GE, Piddock LJ. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014; 9:1165–77.

7). Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006; 19:382–402.

8). Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu Rev Biochem. 2004; 73:467–89.

9). Piddock LJ. Multidrug-resistance efflux pumps – not just for resistance. Nat Rev Microbiol. 2006; 4:629–36.

11). Folster JP, Shafer WM. Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J Bacteriol. 2005; 187:3713–20.
12). Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003; 67:593–656.

13). Olliver A, Vallé M, Chaslus-Dancla E, Cloeckaert A. Role of an acrR mutation in multidrug resistance of in vitro-selected fluoroquinolone-resistant mutants of Salmonella enterica serovar Typhimurium. FEMS Microbiol Lett. 2004; 238:267–72.
14). Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002; 46:3133–41.

15). Wolter DJ, Smith-Moland E, Goering RV, Hanson ND, Lister PD. Multidrug resistance associated with mexXY expression in clinical isolates of Pseudomonas aeruginosa from a Texas hospital. Diagn Microbiol Infect Dis. 2004; 50:43–50.
16). Thanassi DG, Cheng LW, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997; 179:2512–8.
17). Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993; 175:7363–72.
18). Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, et al. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996; 21:713–24.
19). Piddock LJ, White DG, Gensberg K, Pumbwe L, Griggs DJ. Evidence for an efflux pump mediating multiple antibiotic resistance in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother. 2000; 44:3118–21.
20). Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA. 1998; 95:1829–33.
21). Lacroix FJ, Cloeckaert A, Gré pinet O, Pinault C, Popoff MY, Waxin H, et al. Salmonella typhimurium acrB-like gene: identification and role in resistance to biliary salts and detergents and in murine infection. FEMS Microbiol Lett. 1996; 135:161–7.

22). Buckley AM, Webber MA, Cooles S, Randall LP, La Ragione RM, Woodward MJ, et al. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell Microbiol. 2006; 8:847–56.
23). Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun. 2003; 71:5576–82.

24). Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun. 2003; 71:4250–9.
25). Padilla E, Llobet E, Domé nech-Sá nchez A, Martí nez-Martí nez L, Bengoechea JA, Albertí S. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother. 2010; 54:177–83.
26). Pé rez A, Poza M, Ferná ndez A, Ferná ndez Mdel C, Mallo S, Merino M, et al. Involvement of the AcrAB-TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae. Antimicrob Agents Chemother. 2012; 56:2084–90.
27). Hirakata Y, Srikumar R, Poole K, Gotoh N, Suematsu T, Kohno S, et al. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med. 2002; 196:109–18.
28). Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1998; 180:5443–7.
30). Linares JF, Ló pez JA, Camafeita E, Albar JP, Rojo F, Martí nez JL. Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa. J Bacteriol. 2005; 187:1384–91.
31). Rahmati S, Yang S, Davidson AL, Zechiedrich EL. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol Microbiol. 2002; 43:677–85.

32). Magnet S, Courvalin P, Lambert T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob Agents Chemother. 2001; 45:3375–80.
33). Coyne S, Rosenfeld N, Lambert T, Courvalin P, Pé richon B. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2010; 54:4389–93.
34). Damier-Piolle L, Magnet S, Bré mont S, Lambert T, Courvalin P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother. 2008; 52:557–62.
35). Marchand I, Damier-Piolle L, Courvalin P, Lambert T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother. 2004; 48:3298–304.
36). Lin MF, Lin YY, Lan CY. The Role of the two-component system BaeSR in disposing chemicals through regulating transporter systems in Acinetobacter baumannii. PLoS One. 2015; 10:e0132843.
37). Lin MF, Lin YY, Yeh HW, Lan CY. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 2014; 14:119.

38). Lin L, Ling BD, Li XZ. Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial-resistant clinical isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus complex. Int J Antimicrob Agents. 2009; 33:27–32.
39). Rajamohan G, Srinivasan VB, Gebreyes WA. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J Antimicrob Chemother. 2010; 65:228–32.
40). Chu YW, Chau SL, Houang ET. Presence of active efflux systems AdeABC, AdeDE and AdeXYZ in different Acinetobacter genomic DNA groups. J Med Microbiol. 2006; 55:477–8.
41). Chau SL, Chu YW, Houang ET. Novel resistance-nodulation-cell division efflux system AdeDE in Acinetobacter genomic DNA group 3. Antimicrob Agents Chemother. 2004; 48:4054–5.
42). Uroz S, Dessaux Y, Oger P. Quorum sensing and quorum quenching: the yin and yang of bacterial communication. ChemBioChem. 2009; 10:205–16.

43). Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev. 2001; 25:365–404.

44). Bassler BL. Small talk. Cell-to-cell communication in bacteria. Cell. 2002; 109:421–4.
45). Water CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005; 21:319–46.
46). Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008; 190:3386–92.
47). Bhargava N, Sharma P, Capalash N. Quorum sensing in Acinetobacter: an emerging pathogen. Crit Rev Microbiol. 2010; 36:349–60.
48). Irie Y, Parsek MR. Quorum sensing and microbial biofilms. Curr Top Microbiol Immunol. 2008; 322:67–84.

49). Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009; 4:273–8.
50). Prashanth K, Vasanth T, Saranathan R, Makki AR, Sudhakar P. Antibiotic resistance, biofilms and quorum sensing in Acinetobacter species. Dr. Marina P, editor. Antibiotic resistant bacteria – A coninuous challenge in the new millennium. IntechOpen;2012. p. 179–212.

51). Oh MH, Choi CH. Role of LuxIR Homologue AnoIR in Acinetobacter nosocomialis and the Effect of Virstatin on the Expression of anoR Gene. J Microbiol Biotechnol. 2015; 25:1390–400.
52). Gonzá lez RH, Dijkshoorn L, Van den Barselaar M, Nudel C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Rev Argent Microbiol. 2009; 41:73–8.
53). Gonzá lez RH, Nusblat A, Nudel BC. Detection and characterization of quorum sensing signal molecules in Acinetobacter strains. Microbiol Res. 2001; 155:271–7.
54). He X, Lu F, Yuan F, Jiang D, Zhao P, Zhu J, et al. Biofilm formation caused by clinical Acinetobacter baumannii isolates Is associated with overexpression of the AdeFGH efflux pump. Antimicrob Agents Chemother. 2015; 59:4817–25.
55). Kvist M, Hancock V, Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol. 2008; 74:7376–82.

56). Richmond GE, Evans LP, Anderson MJ, Wand ME, Bonney LC, Ivens A, et al. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. MBio. 2016; 7:e00430–16.

57). Dou Y, Song F, Guo F, Zhou Z, Zhu C, Xiang J, et al. Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol Med Rep. 2017; 15:4061–8.

58). Subhadra B, Kim J, Kim DH, Woo K, Oh MH, Choi CH. Local repressor AcrR regulates AcrAB efflux pump required for biofilm formation and virulence in Acinetobacter nosocomialis. Front Cell Infect Microbiol. 2018; 8:270.

59). Chen G, Swem LR, Swem DL, Stauff DL, O'Loughlin CT, Jeffrey PD, et al. A strategy for antagonizing quorum sensing. Mol Cell. 2011; 42:199–209.

61). Roy V, Adams BL, Bentley WE. Developing next generation antimicrobials by intercepting AI-2 mediated quorum sensing. Enzyme Microb Technol. 2011; 49:113–23.

62). Pannek S, Higgins PG, Steinke P, Jonas D, Akova M, Bohnert JA, et al. Multidrug efflux inhibition in Acinetobacter baumannii: comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide. J Antimicrob Chemother. 2006; 57:970–4.
63). Ma Z, Cai SX, Tong WC, Ruan SC, Wang H. Role of the AdeABC efflux pump in carbapenems resistance of clinical isolates of Acinetobacter baumannii. Nan Fang Yi Ke Da Xue Xue Bao. 2011; 31:1378–81.
64). Kuo HY, Chang KC, Kuo JW, Yueh HW, Liou ML. Imipenem: a potent inducer of multidrug resistance in Acinetobacter baumannii. Int J Antimicrob Agents. 2012; 39:33–8.
65). Ribera A, Ruiz J, Jiminez de Anta MT, Vila J. Effect of an efflux pump inhibitor on the MIC of nalidixic acid for Acinetobacter baumannii and Stenotrophomonas maltophilia clinical isolates. J Antimicrob Chemother. 2002; 49:697–8.
66). Mullié C, Guiheneuf R, Serra C, Tir Touil-Meddah A, Sonnet P. Efflux pumps in Acinetobacter baumannii: role in antibiotic resistance and interest of efflux pump inhibitors as additional therapeutic weapons. In: Mé ndez-Vilas A, editor. Microbiology Book Series #6: “ Antimicrobial research: Novel bioknowledge and educational programs”. Formatex Research Center;. 2017. 572–83.
67). Banoee M, Seif S, Nazari ZE, Jafari-Fesharaki P, Shahverdi HR, Moballegh A, et al. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B Appl Biomater. 2010; 93:557–61.
68). Padwal P, Bandyopadhyaya R, Mehra S. Polyacrylic acid-coated iron oxide nanoparticles for targeting drug resistance in mycobacteria. Langmuir. 2014; 30:15266–76.

69). Christena LR, Mangalgowri V, Pradheeba P, Ahmed KB, Shalini BI, Vidyalakshmi M, et al. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RCS Adv. 2015; 5:12899–909.

70). Varga ZG, Armada A, Cerca P, Amaral L, Mior Ahmad Subki MA, Savka MA, et al. Inhibition of quorum sensing and efflux pump system by trifluoromethyl ketone proton pump inhibitors. In Vivo. 2012; 26:277–85.
71). Chang TY, Huang BJ, Sun JR, Perng CL, Chan MC, Yu CP, et al. AdeR protein regulates adeABC expression by binding to a direct-repeat motif in the intercistronic spacer. Microbiol Res. 2016; 183:60–7.
72). Raczkowska A, Trzos J, Lewandowska O, Nieckarz M, Brzostek K. Expression of the AcrAB components of the AcrAB-TolC multidrug efflux pump of Yersinia enterocolitica is subject to dual regulation by OmpR. PLoS One. 2015; 10:e0124248.
Go to : 
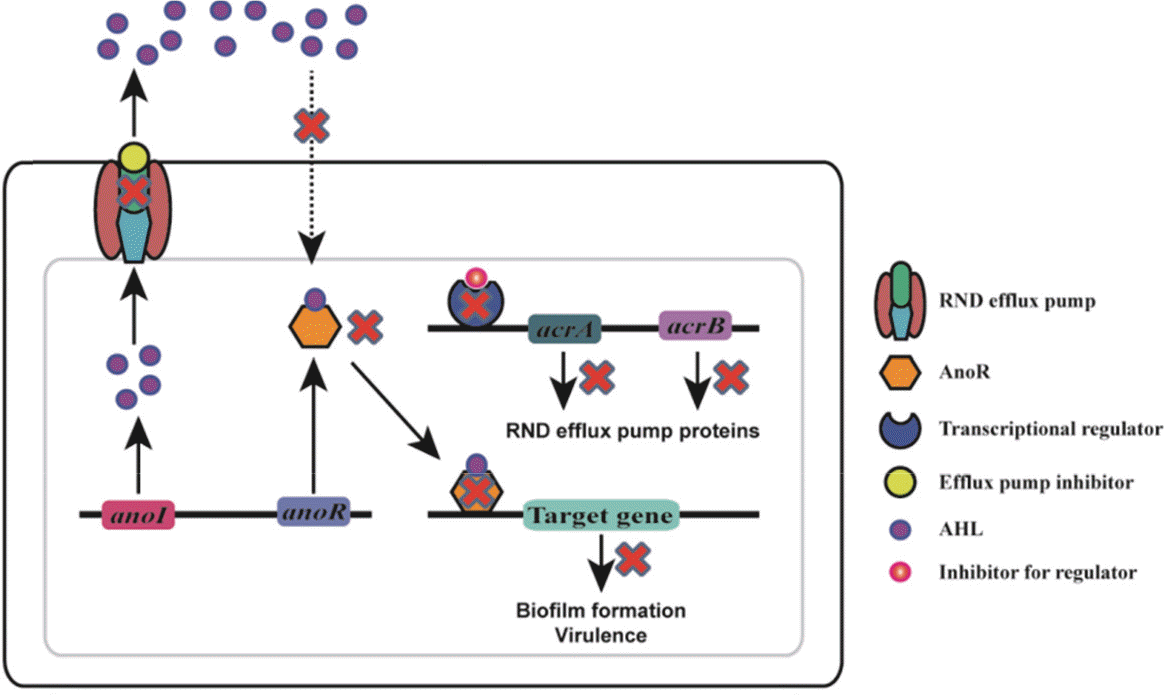 | Figure 1.Schematic representation of quorum quenching strategies targeting multidrug efflux pumps, considering A. nosocomialis as a representative strain. anol and anoR genes encode AHL signal synthase and AHL receptor/activator protein respectively. The efflux pump inhibitors binding to the efflux pumps inhibit the export of AHLs to the external environment blocking the quorum sensing system. The nearby bacterial cells are deprived of AHLs to be transported into the cells disrupting the formation of AnoR-AHL complex, thus preventing the binding of AnoR-AHL to the promoter regions of target genes including those for biofilm formation and virulence. The acrA and acrB efflux pump genes are shown as examples of multidrug efflux pump genes in A. nosocomialis. The biomolecules which target the transcriptional regulators of efflux pump genes inhibit the expression of these genes leading to the disruption of the quorum sensing system. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download