INTRODUCTION
Materials And Methods
Trial design
The World Medical Association's Declaration of Helsinki
Clinical Investigation of Medical Devices for Human Subjects-Good Clinical Practice (ISO 4155:2011)
Guidelines of Good Clinical Practice (2001/20/EC).
Participants
Age over 18 years, as the participants had to be legally competent.
The presence of a tooth or existing implant directly adjacent to the tooth to be extracted.
The absence of a detectable primary need for additional augmentation due to advanced vertical bone defects.
Adequate mouth opening to permit the future insertion of the implant with an implant drilling template.
Non-smoker status or smoking fewer than 10 cigarettes/day.
No administration of bisphosphonates.
No pregnancy.
No alcohol or drug abuse.
No infectious disease, such as hepatitis or human immunodeficiency virus (HIV) and/or acquired immunodeficiency syndrome (AIDS).
No uncontrolled severe diabetes mellitus. In patients with diabetes, the long-term hemoglobin A1c level was required to be below 6.7%.
Interventions
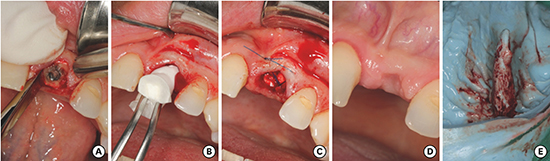
Figure 1
(A) Atraumatic tooth extraction with periotomes and forceps. (B) Introduction of the collagen cone with the membrane. (C) Temporary sutures to stabilize the combination material. (D) Status 3 months after implantation. (E) Impression of the alveolar cavity immediately after tooth extraction.

Avoid eating until the anesthetic effect subsides.
Abstain completely from alcohol, coffee, and caffeinated drinks and cigarettes or other smoking products.
Avoid rinsing the extraction wound to keep the blood clot in place.
Avoid manual manipulation of the wound (e.g., pulling the lip, rigorous cleaning of the wound, etc.).
Outcomes
Digitalization of the impression
Creation of surface models and reprocessing and matching of the data
Figure 4
(A) Exposed surface of the post-extraction alveolus at time T0, immediately after tooth extraction. (B) Exposed surfaces from the impression at time T0 superimposed on the cone-beam computed tomography image (T1) at 8 (±1) weeks post extraction. (C) Specification of measurement points for the sextant measurements. (D) By using the intersections of the diagonal of the measuring aid and the 2 boundary curves, corner points were defined for the creation of 2 individual hexagons. (E) The distance of these constructed corner points was measured in the vertical direction. (★ to ★).
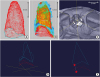
Boundary curve analysis
Figure 5
Three-dimensional measurements of the distances using boundary curves. Based on the 2 applied edge curves, the boundary curve difference was calculated. In each case, a lot was cut from the edge curve of alveolar impression at T0 on the edge curve of the cone-beam computed tomography image of the alveolus at T1. The resulting quantitative results provided the maximum deviation of 2 boundary curves, the mean, and the standard deviation.

Sample size
Randomization
Blinding
Statistical methods
Results
Figure 6
Flow diagram of this randomized trial comparing extraction alone to a ridge-preserving procedure for the treatment of alveolar bone after tooth removal.
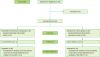
Table 1
Bone degeneration after tooth extraction in the ARP group and control group, including the maxima, medians, and 25th and 75th percentiles

Figure 7
Significantly less bone degeneration was found along the buccal distance in the ARP group (mm).

Table 2
Bone degradation after tooth extraction in the ARP group and control group

Figure 8
Almost complete destruction of the buccal bone was noted at 8 weeks after tooth extraction.

Table 3
Bone degradation after tooth extraction in the ARP group and control group

Table 4
Post hoc power analysis and sample size calculation for the parameters

Table 5
Post hoc power analysis and sample size calculation for the parameters

| Parameter | Effect size (d) | Power (1−β) | Total sample size |
|---|---|---|---|
| Maximum values of the curve | 0.903 | 0.522 | 42 |
| Mean values of the curve | 0.833 | 0.460 | 48 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download