1. International Agency for Research on Cancer. World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide in 2012 [Internet]. Lyon: IARC;2014. cited 2018 Jan 31. Available from:
http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
2. Shim SH, Kim H, Sohn IS, Hwang HS, Kwon HS, Lee SJ, et al. Nationwide cervical cancer screening in Korea: data from the National Health Insurance Service Cancer Screening Program and National Cancer Screening Program, 2009–2014. J Gynecol Oncol. 2017; 28:e63.

3. Ryu HS, Kang SB, Kim KT, Chang KH, Kim JW, Kim JH. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: results of a multicenter retrospective Korean study (KGOG-1005). Int J Gynecol Cancer. 2007; 17:132–136.

4. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18:1606–1613.

5. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999; 73:177–183.

6. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.
7. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

8. Shim SH, Kim SN, Jung PS, Dong M, Kim JE, Lee SJ. Impact of surgical staging on prognosis in patients with borderline ovarian tumours: a meta-analysis. Eur J Cancer. 2016; 54:84–95.

9. In : Wells GA, Shea B, O'connell D, Peterson J, Welch V, Losos M, editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000 Jul 3–5; Oxford, United Kingdom. Ottawa: Ottawa Hospital Research Institute;2000.
10. Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006; 13:413–424.

11. OuYang PY, Xie C, Mao YP, Zhang Y, Liang XX, Su Z, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol. 2013; 24:2136–2146.

12. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.

13. Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992; 14:154–176.

14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188.

16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.

17. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001; 54:1046–1055.
18. Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983; 8:157–159.

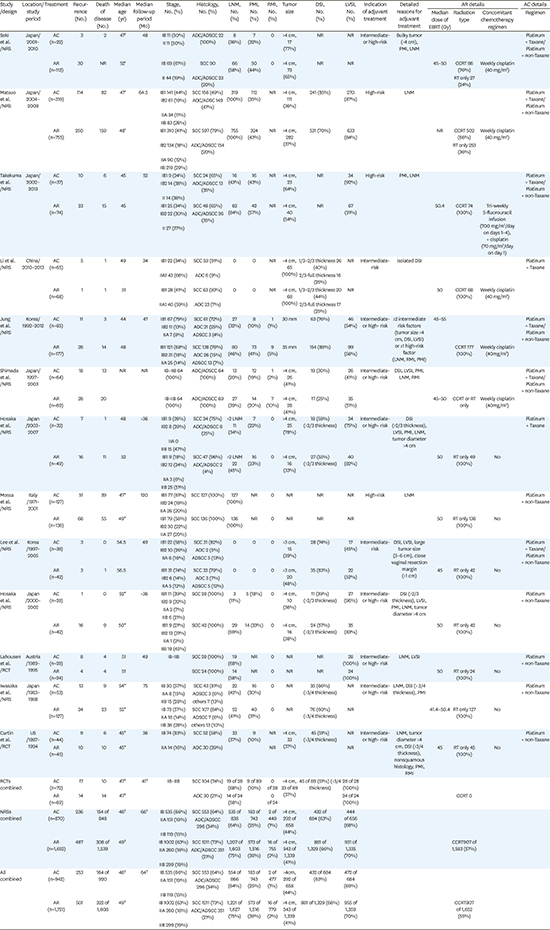
19. Seki T, Tanabe H, Nagata C, Suzuki J, Suzuki K, Takano H, et al. Adjuvant therapy after radical surgery for stage IB-IIB cervical adenocarcinoma with risk factors. Jpn J Clin Oncol. 2017; 47:32–38.

20. Matsuo K, Shimada M, Aoki Y, Sakamoto M, Takeshima N, Fujiwara H, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer. 2017; 141:1042–1051.

21. Takekuma M, Kasamatsu Y, Kado N, Kuji S, Tanaka A, Takahashi N, et al. Adjuvant chemotherapy versus concurrent chemoradiotherapy for high-risk cervical cancer after radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol. 2016; 21:741–747.

22. Li L, Song X, Liu R, Li N, Zhang Y, Cheng Y, et al. Chemotherapy versus radiotherapy for FIGO stages IB1 and IIA1 cervical carcinoma patients with postoperative isolated deep stromal invasion: a retrospective study. BMC Cancer. 2016; 16:403.

23. Jung PS, Kim DY, Lee SW, Park JY, Suh DS, Kim JH, et al. Clinical role of adjuvant chemotherapy after radical hysterectomy for FIGO stage IB-IIA cervical cancer: comparison with adjuvant RT/CCRT using inverse-probability-of-treatment weighting. PLoS One. 2015; 10:e0132298.

24. Shimada M, Nishimura R, Hatae M, Hiura M, Takehara K, Tase T, et al. Comparison of adjuvant chemotherapy and radiotherapy in patients with cervical adenocarcinoma of the uterus after radical hysterectomy: SGSG/TGCU Intergroup surveillance. Eur J Gynaecol Oncol. 2013; 34:425–428.
25. Hosaka M, Watari H, Takeda M, Moriwaki M, Hara Y, Todo Y, et al. Treatment of cervical cancer with adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy and systematic lymphadenectomy. J Obstet Gynaecol Res. 2008; 34:552–556.

26. Mossa B, Mossa S, Marziani R. Adjuvant chemotherapy versus radiation therapy after radical surgery in high-risk positive node stage IB/IIA cervical cancer. Eur J Gynaecol Oncol. 2010; 31:545–550.
27. Lee KB, Lee JM, Ki KD, Lee SK, Park CY, Ha SY. Comparison of adjuvant chemotherapy and radiation in patients with intermediate risk factors after radical surgery in FIGO stage IB-IIA cervical cancer. Int J Gynecol Cancer. 2008; 18:1027–1031.

28. Hosaka M, Watari H, Kato T, Odagiri T, Konno Y, Endo D, et al. Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol. 2012; 105:612–616.

29. Lahousen M, Haas J, Pickel H, Hackl A, Kurz C, Ogris H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: a randomized, prospective, multicenter trial. Gynecol Oncol. 1999; 73:196–201.

30. Iwasaka T, Kamura T, Yokoyama M, Matsuo N, Nakano H, Sugimori H. Adjuvant chemotherapy after radical hysterectomy for cervical carcinoma: a comparison with effects of adjuvant radiotherapy. Obstet Gynecol. 1998; 91:977–981.

31. Curtin JP, Hoskins WJ, Venkatraman ES, Almadrones L, Podratz KC, Long H, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH-PLND): a randomized phase III trial. Gynecol Oncol. 1996; 61:3–10.

32. Look KY, Brunetto VL, Clarke-Pearson DL, Averette HE, Major FJ, Alvarez RD, et al. An analysis of cell type in patients with surgically staged stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1996; 63:304–311.

33. Balbergen A, Ewing-Graham PC, Hop WC, Struijk P, Helmerhorst TJ. Prognostic factors in adenocarcinoma of the uterine cervix. Gynecol Oncol. 2004; 92:262–267.
34. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer. 2010; 102:1692–1698.

35. Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012; 125:287–291.

36. Hopkins MP, Peters WA 3rd, Andersen W, Morley GW. Invasive cervical cancer treated initially by standard hysterectomy. Gynecol Oncol. 1990; 36:7–12.

37. Lee KB, Kim YS, Lee JM. Oncologic outcomes of adjuvant chemotherapy alone after radical surgery for stage IB-IIA cervical cancer patients. J Gynecol Oncol. 2018; 29:e5.

39. Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014; 370:734–743.

40. Yamaguchi S, Nishimura R, Yaegashi N, Kiguchi K, Sugiyama T, Kita T, et al. Phase II study of neoadjuvant chemotherapy with irinotecan hydrochloride and nedaplatin followed by radical hysterectomy for bulky stage Ib2 to IIb, cervical squamous cell carcinoma: Japanese Gynecologic Oncology Group study (JGOG 1065). Oncol Rep. 2012; 28:487–493.








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download