Abstract
Intraoperative pulmonary thromboembolism is a high mortality situation. Early mortality in patients with pulmonary thromboembolism varies from 2% in normotensive patients to 30% in patients with cardiogenic shock. The use of extracorporeal cardiopulmonary resuscitation can improve survival and neurologic outcomes of cardiac arrest. We report a case of intraoperative massive pulmonary thromboembolism with circulatory collapse and cardiac arrest during anesthesia for pelvic bone fracture surgery, which were rescued by extracorporeal membrane oxygenation.
An intraoperative pulmonary thromboembolism is hard to diagnose and a lethal disease that could lead to the death. In addition, cardiac arrest caused by this makes it difficult to revive the patient due to the difficulty of removing its cause, the pulmonary thromboembolism. Cardiopulmonary resuscitation using extracorporeal membrane oxygenation which was introduced in 1950s and showed higher survival rate when performed in general cardiopulmonary resuscitation.1 The authors would like to report our experience with cardiopulmonary resuscitation using an extracorporeal membrane oxygenation device on a patient in a cardiac arrest state caused by pulmonary thromboembolism during orthopedic surgery.
A male patient who was 72 years old, 158 cm in height and 74 kg fell from a 3 m height and was admitted to the orthopedic department with the main complaint being acetabular fracture and planned for open taxis and internal fixation. He had a history of hypertension and had been taking medicine for 5 years. There was no other specific history.
After being admitted, no abnormalities in the blood test, coagulation test, and chemical test were found. The lung function test, echocardiogram, ECG, and chest radiograph taken 5 days prior to the surgery all came out normal. In the arterial blood gas test taken right before the surgery there were no abnormalities with the pH level at 7.440, pCO2 at 32 mmHg, pO2 at 84 mmHg, HCO3− at 21.7mmol/L, and the oxygen saturation was at 97%. From admittance to the day of surgery, the patient received traction therapy, and the pain was controlled while resting in bed.
The FDP, D-dimer test Administered the Day Before Surgery had Normal Results
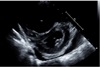
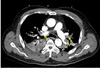
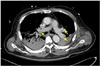
On the day of the surgery, after entering the operating room, a 20G catheter was inserted in the right radial artery and the arterial pressure was observed while an 18G IV line was secured in the left upper arm. 0.5% bupivacaine 12 mg, fentanyl 10mcg were mixed and injected into the subarachnoid space between vertebrae 3 and 4 in the lateral decubitus position. The patient was returned to a supine position. After checking for a blockage around T10, the operation began. Following the spinal anesthesia, the time from operation preparation to the start of the operation was around 1 hour. The arterial blood gas test taken after the start of the surgery was measured to be Hb 8.8g/dL so 1unit of RBC was administered through intravenous infusion. At 60 minutes into the surgery, due to a complaint of pain in the area being operated on, 300mg of thiopental and 7mg of vecuronium was administered through intravenous infusion while using sevoflurane for general anesthesia and a tracheal intubation was performed. Immediately after tracheal intubation, oxygen saturation, CO2 partial pressure, systolic and diastolic blood pressures could not be measured so CPR (10–20 rpm) was performed. Premature ventricular complex (PVC) was observed so they began cardiopulmonary resuscitation. While monitoring the arterial pressure, chest compressions were performed to keep the systolic blood pressure at 80–100 mmHg. A total of 12 injections of epinephrine were injected every 3 minutes and 5 injections of atropine due to bradycardia. Epinephrine was started at 0.05 mcg / kg / min. Following 40 minutes of cardio pulmonary resuscitation, the patient's blood pressure and pulse did not spontaneously recover. The patient's arterial blood was tested and was measured to have pH 7.18, pCO2 67 mmHg, pO2 30 mmHg, and HCO3− 25.0 mmol / L. In the meantime, the orthopedic surgeon urgently finished the surgery. 40 minutes after cardiopulmonary resuscitation, ECMO (O2 5 L / min, FiO2 1.0, RPM 1980, Flow 3.7 Cardiac index 2.1) was applied to the right femoral vein and artery by extracorporeal membrane oxygenation (ECMO). After ECMO insertion, the patient's systolic blood pressure was over 150 mmHg and 3 to 5 ventricular premature contractions per minute with normal sinus rhythm was seen. In the arterial blood gas test, pH 7.20, pO2 519 mmHg, pCO2 41 mmHg and HCO3− 16 mmol / L were measured. Soon after, an internal medicine doctor with specialization in circulatory system performed an echocardiogram where a right ventricular hypertrophy and D-shaped left ventricular function (Fig. 1) were seen and pulmonary thromboembolism was suspected to be the cause for cardiac arrest. A chest computed tomography (CT) scan of the left ventricular endothelial lumen was performed to confirm the presence of a massive bilateral pulmonary thromboembolism that blocked the left and right pulmonary arteries and all branches (Fig. 2). Later in the intensive care unit, ECMO and activated partial thromboplastin time were maintained for 60 – 80 seconds, and the hepatic thromboembolism was treated with heparin. The thoracic computed tomography (CT) performed on the 13th day following the surgery showed a decrease in pulmonary thromboembolism (Fig. 3). An infection at the ECMO insertion site (fever, redness) was seen, but it improved through the use of antibiotics and disinfection. On the 19th day after the operation, the ECMO flow was at 2.19 RPM 2285 and normal cardiac function was seen so the ECMO was removed. On the 31st day after the operation, an arterial blood gas test was performed and measured the pH 7.35, pO2 123 mmHg, pCO2 43 mmHb, and HCO3− 23 mmol / L. The patient was discharged without any neurological damage.
In general surgery, pulmonary thromboembolism occurs at a frequency of 0.3 to 1.6%. Pulmonary thromboembolism occurs in 4.3–24% of cases in orthopedic surgery, and is especially seen in pelvic fracture and femoral fracture surgery. Among the cases hemodynamic instability occurs in 3.6 – 12.9% of cases.2 The risk factors for pulmonary thromboembolism include deep vein thrombosis of the lower limb, lying on a bed for an extended period, obesity, malignancy, aging, and lower extremity fracture. The most highly recommended method for the prevention of pulmonary thromboembolism in patients with pelvic fractures is to combine low molecular weight heparin with mechanical interventions such as intermittent pneumatic compression pumps (IPC pumps) and grafted compression stockings (GCSs). 3 In patients with a high-risk for lower-limb venous thrombosis, the use of IVC filters is recommended when low-molecular-weight heparin cannot be used due to bleeding tendency.3
However, in this patient, the IPC pump was used as a precautionary measure due to the risk of postoperative bleeding. Lethal pulmonary thromboembolism was thought to have been caused by various risk factors such as the pelvic bone fracture, long-term bed confinement, and obesity.
In the event of cardiac arrest due to pulmonary thromboembolism, the patient could have been treated with a streptokinase or recombinant tissue plasminogen activator. However, during the operation, pulmonary thromboembolism could not be confirmed. In the case of pelvic fracture repair, it was judged that it was appropriate to use an extracorporeal membrane oxygenator because it was expected that there would be a lot of bleeding. In addition, surgical removal of the pulmonary thromboembolism was also considered. Through the use of the ECMO, the blood pressure and oxygen saturation were maintained, and the thrombectomy with heparin was performed.
During general anesthesia, it is recommended the patient undergo transesophageal echocardiography to determine the cause of cardiac arrest. In this patient, a more rapid transesophageal echocardiography would have shown the cause of the cardiac arrest and given doctors a chance to respond to the cardiac arrest.
ECMO is a device that was designed in the 1950s and has recently been widely used as an aid to cardiopulmonary function in patients with cardiac and pulmonary insufficiency. In addition, the concept of CPR using ECMO (ECPR) has emerged in situations requiring cardiopulmonary resuscitation and cardiopulmonary resuscitation.4 Recent studies have shown that ECPR leads to better survival (30-day survival, 1-year survival) than conventional CPR without ECMO.1
The ECPR distributed by the ELSO can treat cardiac arrest caused by reversible causes and also cardiac arrest that has undergone effective cardiopulmonary resuscitation prior to the use of the ECMO.5 Furthermore, ECPR is ideally performed within 30 minutes after cardiac arrest, but it may be administered up to 60 minutes after.46 In this patient, it took 40 minutes to apply ECMO. However, CPR was performed immediately after cardiac arrest and effective cardiopulmonary resuscitation was performed by monitoring arterial pressure. Therefore, after ECMO application the patient recovered without neurological damage. It is clearly stated that the ECPR tabled by the Extracorporeal Life Support Organization should not be used in the case of unrecoverable cardiac arrest caused by long-term dysfunction such as old age, liver, kidney, lung, and cardiopulmonary resuscitation that does not provide adequate oxygen supply.5 If this is not the case, ECPR should be actively considered.
ELSO as a result of ECMO weaning. 1) Recovery of arterial pulse 2) Recovery of myocardial contraction by echocardiography 3) Reduction of ECMO flow by 50% or more 4) In the case that the blood pressure is maintained after the ECMO was clamped.5
In cases reported in other journals, ECPR was performed in cardiac arrest due to myocardial infarction, Eisenmenger syndrome, and malignant hyperthermia, and patients were resected. Therefore, active ECPR may be considered when these diseases are suspected during surgery.
Positive prognostic factors for ECPR reported in the meta-analyses of 841 patients include: 1) shockable cardiac rhythm (ventricular tachycardia, ventricular tachycardia), 2) short low-flow duration (from the onset of CPR to the onset of ECPR Time) 3) High arterial pH 4) Low serum lactate level.6 In this patient's case, the pH was 7.18 at the time of cardiac arrest and the serum lactate level was less than 2.0 mmol / L.
It should be kept in mind that in cardiopulmonary resuscitation (CPR) as well as general CPR in cardiopulmonary resuscitation during surgery that ECMO can also be used. It should be noted that through the application of rapid ECPR there was a good prognosis for the patient.
Figures and Tables
References
1. Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest. an observational study and propensity analysis. Lancet. 2008; 372:554–561.

2. Geerts WH, Bergqvist D, Pineo GF, et al. American College of Chest Physicians. Prevention of venous thromboembolism. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:6 Suppl. 381S–453S.
3. Chana-Rodríguez F, Mañanes RP, Rojo-Manaute J, Haro JA, Vaquero-Martín J. Methods and Guidelines for Venous Thromboembolism Prevention in Polytrauma Patients with Pelvic and Acetabular Fractures. Open Orthop J. 2015; 9:313–320.

4. Conrad SA, Rycus PT. Extracorporeal membrane oxygenation for refractory cardiac arrest. Ann Card Anaesth. 2017; 20:S4–S10.

5. ELSO guidelines. Extracorporeal life support organization, version 1.3. ANN Arbor: USA;2013. 11. available at: https://www.elso.org/resources/guidelines.aspx.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download