Abstract
Hairy cell leukemia (HCL) is a rare chronic B cell leukemia morphologically characterized by cells with an abundant cytoplasm and hair-like projections that can be found in the peripheral blood and bone marrow. The treatment for HCL is splenectomy or chemotherapy with the purine analogs pentostatin and cladribine. However, patients continue to relapse. Retreatment with the same or alternate purine analogs produces lower response rates and a shorter duration of response. Fludarabine is another purine analog widely used in treating indolent lymphoid cancers, often in combination with rituximab. Here, we report a case of HCL variant in a 60-year-old man who experienced multiple relapses after splenectomy and retreatment with cladribine. The patient was then treated with fludarabine and rituximab combination chemotherapy. After the treatment, he achieved complete remission that continued for 35 months.
Hairy cell leukemia (HCL) is a relatively rare chronic B cell leukemia accounting for 2% of leukemia cases. It is characterized by the accumulation of small mature B cell lymphoid cells with an abundant cytoplasm and “hairy” projections found in the peripheral blood, bone marrow, and splenic red pulp. HCL variant (HCL-V) has similar clinical features of classic HCL but only infrequently includes reactivity for tartrate-resistant acid phosphatase (TRAP). The majority of patients do not require therapy immediately upon diagnosis. Treatment is reserved until symptomatic or threatening progression of HCL. In practice, treatment is initiated at the onset of neutropenia, thrombocytopenia, anemia resulting in increased risk of infection or bleeding, symptomatic anemia, or symptomatic splenomegaly.1 A number of treatment options are available for patients with symptomatic HCL including splenectomy, interferon-alpha, and cytotoxic chemotherapy with the purine analogs cladribine and pentostatin. However, patients relapse in 30% to 40% of cases.2 Retreatment with the same or alternate purine analog produces lower response rates and a shorter duration of response.2 Thus, alternative treatment approaches are needed for this disease (e.g. more novel immunotherapies and targeted kinase inhibitors). Although treatment data are sparse, patients with relapsed or refractory HCL are candidates for treatment with monoclonal antibodies (e.g. rituximab, BL-22, or alemtuzumab). Rituximab has been found to increase the sensitivity of malignant cells to purine analogs, suggesting that combination therapy may improve the treatment response in cases of HCL. Another purine analog, fludarabine, is used to treat indolent lymphoid cancers, often in combination with rituximab, but there are few reports of its use for HCL. Here we report the case of a patient with HCL who relapsed multiple times after a splenectomy and cladribine treatments. The patient received fludarabine and rituximab chemoimmunotherapy and reached complete remission. As of a follow-up examination at 35 months, the patient remains in remission.
A 60-year-old male visited the emergency department in our hospital with left side weakness, dysarthria, diplopia, and petechiae on both thighs that began 7 days prior. He had no relevant past medical or family history except hypertension and hyperlipidemia. Constitutional symptoms such as weight loss, fever, or night sweats were absent. Physical examination revealed mild splenomegaly, but no palpable enlarged lymph nodes. The complete blood count (CBC) revealed a hemoglobin (Hb) concentration of 11.2 g/dL and a white blood cell (WBC) count of 11.3 × 103/µL with 60.2% lymphocytes, and a decreased platelet count of 4.0 × 103/µL. A computerized tomography (CT) scan and MRI of the brain revealed a right pontine hemorrhage. An abdominopelvic CT scan and abdominopelvic sonography revealed splenomegaly (12.5 cm in length) with no other specific findings. A peripheral blood smear (PB) demonstrated a few atypical cells with a light basophilic cytoplasm containing numerous hairy projections on the outer surface. The PB demonstrated hairy cells (Fig. 1A, B). Bone marrow aspirates were easily obtained on the first attempt and serrated cytoplasms could be seen (Fig. 1C, D). Hairy cells were minimally infiltrated into some areas. Focal fibrosis was noted (Fig. 1E, F). Immunophenotypical testing was positive for CD19, CD20, CD22, without CD25. Tartrate-resistant acid phosphatase (TRAP) was negative. On cytogenetic analysis, a chromosomal abnormality was detected (45,XY,der(13;14)(q10;q10)). Based on the clinical, peripheral smear, bone marrow, and immunohistochemical studies, a diagnosis of HCL-V was confirmed.
After clinical improvement, the patient underwent a splenectomy. Ten days after the splenectomy the patient's platelet count rose to 581 × 103/µL due to reactive thrombocytosis (Fig. 2). After four months, his platelet count decreased to 31 × 103/µL and a follow-up bone marrow biopsy was done to confirm his relapse. Compared with the previous testing, his bone marrow biopsy revealed a slightly increased proportion of hairy cells and a marked increase in cellularity and fibrosis. The patient received cladribine retreatment and complete remission was observed. On a follow-up bone marrow biopsy there was no evidence of abnormal localization of hairy cells. However, after 2 courses of cladribine therapy, the patient experienced prolonged pancytopenia due to chemotherapy-induced myelosuppression.
After a period of 22 months without disease progression, his HCL relapsed and the patient received cladribine retreatment. After about 2 years he had severe thrombocytopenia and a bone marrow biopsy revealed that his HCL had again relapsed. Therefore, we used 8 cycles of fludarabine (25 mg/m2) on day 1 to day 5 and rituximab (375 mg/m2) on day 1 as a fourth-line treatment option. The disappearance of hairy cells from the peripheral blood was observed one day after the first administration of rituximab and fludarabine (FR) combination therapy. During this therapy, toxicity was minimal and no infectious episodes were observed. After 8 cycles of the combination treatment, bone marrow aspiration was performed and no hairy cells were found. The patient experienced complete remission that has continued as of 35 months.
HCL-V is an uncommon chronic B cell lymphoproliferative disorder accounting for 10% – 20% of HCL cases and 0.4% of chronic lymphoid malignancies. The median age of patients is 71. Unlike classic HCL, HCL-V is resistant to most conventional treatments, including splenectomy and purine analogs. Many patients are unresponsive to therapy or relapse. HCL-V remains an incurable disease and the introduction of new drugs and new therapeutic strategies is necessary.
Approximately 90% of HCL patients have splenomegaly, and splenectomy was the first effective treatment for this disease.3 Although splenectomy does not produce pathologic remission, peripheral blood counts return to normal in 40% – 70% of patients and overall 5-year survival rates are 60% – 70%.45 Patients with HCL benefit from splenectomy compared with non-splenectomized patients,6 and they respond better and faster to the following therapy. These results suggest that splenectomy should be considered for patients with HCL because it corrects cytopenias and removes a significant bulk of the disease.7 Currently, the purine nucleoside analogs, like cladribine and pentostatin, are the drugs of choice in the treatment of HCL.8 These drugs induce a similarly high response rate and a longer overall survival in patients with HCL. Additionally, fludarabine, a halogenated deoxyadenosine derivative similar to 2-chlorodeoxyadenosine, is a primary therapeutic option for chronic lymphocytic leukemia. Several case reports have been published describing the activity of fludarabine in patients with HCL.9 Rituximab may be an effective treatment for relapsed or refractory HCL. Several clinical trials indicate that the humanized anti-CD20 monoclonal antibody, rituximab, is active in HCL. Rituximab is a chimeric immunoglobulin G1 (IgG1) κ monoclonal antibody that targets the CD20 antigen expressed on most malignant B cell leukemias and lymphoma. Rituximab induces apoptosis in addition to complementing the antibody-mediated cellular cytotoxicity. It has activity in both low-grade lymphoma and chronic lymphocytic leukemia.101112 Recently, Gerrie et al.1 published a retrospective analysis of 15 patients with relapsed or refractory HCL after different previous treatment attempts, including at least one other purine analog. They were then treated with fludarabine 40 mg/m2 per day orally on five consecutive days in combination with the intravenous injection of 375 mg/m2 rituximab on day 1. This was administered every 28 days for four cycles between 2004 and 2010.1 Without assessing the depth of the response in most of the cases, these authors report well-tolerated, safe, and effective results. After a median follow-up period of 35 months, there were 14 progression-free patients. Their 5-year progression-free survival was 89%, overall survival was 83%, and the recurrence rate was 7%.1 These results may suggest fludarabine and rituximab combination regimen as a therapeutic option for patients with relapsed or refractory HCL.7
In other study, patients who were positive for minimal residual disease (MRD) after treatment with cladribine showed a high level of expression of CD20 with more than 100,000 sites per cell.13 Cells with high levels of CD20 expression represent a target for the humanized anti-CD20 monoclonal antibody (mAB) rituximab.13 A dosage of 375 mg/m2 of rituximab in weekly intravenous infusions is common.1415 However, the optimal dosing schedule has not yet been established. Six to eight infusions may be necessary to reach a complete remission (CR) and MRD eradication.16 A synergistic effect is seen with the combination of rituximab with a purine analog. This combination results in a higher complete remission rate (CRR), up to 100%,15 with MRD eradication up to 92%.17 The combination of rituximab and a purine nucleoside analog is not only effective in newly diagnosed and relapsed cases but also in patients resistant to single use of cladribine and in cases of MRD.1518
Currently, principles for treating this rare disease derive from uncontrolled single-institution studies or single case reports. For these reasons, the outcome of therapy is unsatisfactory with no standard therapy. Unfortunately, randomized controlled trials are not feasible because of the insufficient number of cases.
In conclusion, the combination therapy of fludarabine and rituximab can be given successfully to patients for relapsed or refractory HCL, including those previously exposed to alternate purine analogs. There is no international consensus for a standardized procedure for relapsed, refractory HCL or patients with MRD. More extensive studies on the efficacy and safety of this combination therapy are required to determine its potential as a first line approach for treating HCL.
Figures and Tables
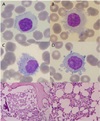 | Fig. 1Bone marrow aspiration and biopsy. This figure depicts the characteristic malignant cell in the peripheral blood and bone marrow from a patient with hairy cell leukemia. Hairy cells with radial projections from their surface are noted in peripheral blood smear (A,B) and bone marrow aspirate (C,D) with Wright's stain. (×1000) The bone marrow biopsy section shows a subtle infiltration of hairy cell leukemia (E,F). Small and reniform and cleaved nuclei are present, surrounded by ample amounts of clear cytoplasm. And focal fibrosis are noted (H&E, ×100, ×400) |
 | Fig. 2The blood counts according to treatments. After splenectomy, platelet recovered within normal range. After first relapse, first course of cladribine chemotherapy showed complete remission(CR) criteria. After second relapse, second course of cladribine chemotherapy also showed complete remission(CR). But the patient experienced myelosuppression due cladribine chemotherapy. After disease free period of 24 months, relapse is revealed by bone marrow biopsy. Fludarabine and rituximab chemotherapy revealed complete remission(CR) from treatment initiation. |
References
1. Gerrie AS, Zypchen LN, Connors JM. Fludarabine and rituximab for relapsed or refractory hairy cell leukemia. Blood. 2012; 119:1988–1991.

2. Else M, Dearden CE, Matutes E, Garcia-Talavera J, Rohatiner AZ, Johnson SA, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009; 145:733–740.

3. Sgarabotto D, Vianello F, Radossi P, Poletti A, Sotti G, Stefani PM, et al. Remission in hairy cell leukemia-variant following splenic radiotherapy alone. Leuk Lymphoma. 1997; 26:395–398.

4. Golomb HM, Vardiman JW. Response to splenectomy in 65 patients with hairy cell leukemia: an evaluation of spleen weight and bone marrow involvement. Blood. 1983; 61:349–352.

5. Magee MJ, McKenzie S, Filippa DA, Arlin ZA, Gee TS, Clarkson BD. Hairy cell leukemia. Durability of response to splenectomy in 26 patients and treatment of relapse with androgens in six patients. Cancer. 1985; 56:2557–2562.

6. Habermann TM, Rai K. Historical treatments of in hairy cell leukemia, splenectomy and interferon: past and current uses. Leuk Lymphoma. 2011; 52:18–20.

7. Maevis V, Mey U, Schmidt-Wolf G, Schmidt-Wolf IG. Hairy cell leukemia: short review, today's recommendations and outlook. Blood Cancer J. 2014; 4:e184.

8. Robak T. Current treatment options in hairy cell leukemia and hairy cell leukemia variant. Cancer Treat Rev. 2006; 32:365–376.

9. Johnston JB. Mechanism of action of pentostatin and cladribine in hairy cell leukemia. Leuk Lymphoma. 2011; 52:43–45.

10. Almasri NM, Duque RE, Iturraspe J, Everett E, Braylan RC. Reduced expression of CD20 antigen as a characteristic marker for chronic lymphocytic leukemia. Am J Hematol. 1992; 40:259–263.

11. Robbins BA, Ellison DJ, Spinosa JC, Carey CA, Lukes RJ, Poppema S, et al. Diagnostic application of two-color flow cytometry in 161 cases of hairy cell leukemia. Blood. 1993; 82:1277–1287.

12. Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994; 83:435–445.

13. Kreitman RJ, Arons E, Stetler-Stevenson M, Fitzgerald DJ, Wilson WH, Pastan I. Recombinant immunotoxins and other therapies for relapsed/ refractory hairy cell leukemia. Leuk Lymphoma. 2011; 52:82–86.

14. Jones G, Parry-Jones N, Wilkins B, Else M, Catovsky D. British Committee for Standards in Haematology. Revised guidelines for the diagnosis and management of hairy cell leukaemia and hairy cell leukaemia variant*. Br J Haematol. 2012; 156:186–195.

15. Malfuson JV, Fagot T, Konopacki J, Souleau B, Cremades S, de Revel T. Which role for rituximab in hairy cell leukemia? Reflections on six cases. Acta Haematol. 2010; 123:110–116.

16. Else M, Dearden CE, Matutes E, Forconi F, Lauria F, Ahmad H, et al. Rituximab with pentostatin or cladribine: an effective combination treatment for hairy cell leukemia after disease recurrence. Leuk Lymphoma. 2011; 52:75–78.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download