Abstract
Central venous catheterization is a routinely performed procedure in clinical practice. While cerebral air embolism after the removal of the central venous catheter is very rare, it is one of the most serious complications that can lead to fatal outcomes. In this report, we present a rare case of a cerebral air embolism after the removal of the central venous catheter in a patient with a patent foramen ovale.
Central venous catheterization is widely performed in critically ill patients. Central venous catheters are usually placed for various purposes, such as hemodynamic monitoring, administration of medication and nutrition, and hemodialysis. When performed accurately and reliably by skilled medical practitioners, central venous catheterization is relatively safe. However, there is always the possibility of various complications. Complications associated with central venous catheterization can occur at any time during insertion, use, and removal of the catheter. Common complications include infection, local hematoma, pneumothorax, and hemothorax. Air embolism is a rare, but potentially fatal complication and is mostly associated with pulmonary embolism. Cerebral air embolism, although very rare, can occur and lead to lethal consequences. To our best knowledge, cerebral air embolism after the removal of the central venous catheter is rare and its occurrence in patient with patent foramen ovale (PFO) is exceptional, with few cases reported in the literature (123). Herein, we present a rare case of cerebral air embolism that developed following the central venous catheter removal in a patient with a PFO.
A 67-year-old woman with diffuse abdominal pain of 3-day duration visited the emergency room of other hospital. Cecal perforation with peritonitis was diagnosed on CT of the abdomen. The patient was transferred to our hospital for surgical treatment, and a repair of the perforated site was successfully performed. After surgery, the patient's vital signs were stable and she was transferred to intensive care unit (ICU) for postoperative care. However, aspiration pneumonia and associated acute respiratory failure developed. The central venous catheter was placed through the patient's right subclavian vein for hemodynamic monitoring and drug administration. After about two months of ICU treatment, the patient improved and the catheter was removed in the supine position using the Valsalva maneuver. However, the patient experienced difficulty in holding her breath, so a proper Valsalva maneuver was impossible during the procedure. In several minutes, the patient showed a decrease in oxygen saturation, no response to stimulation, and bilateral decerebrate posture.
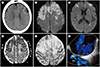
In 1 hour after the onset of symptoms, CT of the brain revealed a focal low attenuation area in the left frontal lobe and tiny air densities in the adjacent area, suggesting acute cerebral infarction, secondary to air embolism (Fig. 1A). MRI of the brain was performed after 29 hours from the onset of the symptoms. Diffusion-weighted imaging (DWI) showed multifocal high signal intensities along both cerebral hemispheres and basal gangli with marked confluent high signal intensities at both frontal lobes (Fig. 1B, C). These abnormal lesions showed low signal intensities on the corresponding apparent diffusion coefficient map (Fig. 1D). Considering the patient's neurologic symptom and CT findings, multiple dark signal dots at both frontal lobes on gradient-echo (GRE) image were suggestive of air embolism (Fig. 1E). However, microbleeds should also be considered as a differential diagnosis. The brain CT and MRI findings suggested that cerebral embolic infarct was caused by air embolism. Transesophageal echocardiography showed a PFO with a cor pulmonale due to the chronic destructive lung disease (Fig. 1F). Finally, a paradoxical cerebral air embolism following the central venous catheter removal was diagnosed. After five months from the initial neurological insult, the patient was discharged with neurological sequelae of being able only to stand because of gait disturbance.
Cerebral air embolism after removal of central venous catheterization is a very rare, but a potentially life-threatening complication. It can occur when air or gas is admitted into the cerebral artery. A paradoxical embolism occurs when air or gas that has been introduced into the venous circulation carries into the systemic arterial circulation and causes symptoms of end-artery obstruction (4).
There are two mechanisms to explain paradoxical cerebral air embolism following the central venous catheter removal. First, there is an intracardiac right-to-left shunt, such as a congenital atrial septal malformation or a PFO, which is known to be the cause of common paradoxical air embolism (1). The overall incidence of a PFO is about 30% in the general population, which can lead to right-to-left shunting of air bubbles (4). If the right atrial pressure exceeds the left atrial pressure, the right-to-left flow may occur through a PFO. Second, air can enter the systemic arterial circulation due to the incomplete filtering of the air in the presence of pulmonary arteriovenous malformations (PAVMs) or intrapulmonary arteriovenous anastomoses in healthy individuals. PAVMs are an abnormal vascular communication between the pulmonary artery and the pulmonary vein leading to an intrapulmonary right-to-left shunt. As a result, a patient with PAVMs may develop paradoxical embolization complications, including stroke and brain abscess (5). PAVMs are also known to be inherited and related to hereditary hemorrhagic telangiectasia. Another cause of transpulmonary air passage is an inducible large diameter intrapulmonary arteriovenous anastomosis. Lovering et al. (6) have demonstrated that these anastomoses are closed at rest, but can open during hyperdynamic conditions, such as exercise in over 90% healthy humans without intracardiac shunt. Therefore, air emboli bypass the pulmonary microcirculation via dynamic large diameter intrapulmonary arteriovenous anastomoses, resulting in embolic infarction. In addition, the fraction of inspired oxygen, body positioning, and underlying lung disease leading to pulmonary hypertension can play a role in controlling the patency of these inducible intrapulmonary arteriovenous anastomoses (67). In our case, since the patient had PFO (Fig. 1F) and the clinical symptoms developed immediately after the removal of the central line, cerebral air embolism is thought to have been caused by a PFO, rather than by transpulmonary air passage.
Various neurological symptoms due to cerebral air embolism appear as different pathophysiological mechanisms depending on the size of air embolism. The large air embolus is considered to be caused by occlusion of the peripheral arterial blood flow and subsequent infarction. On the other hand, the small air embolus is considered to be involved in the expression of an inflammatory reaction of vascular endothelial cells damaged by air bubble contained in the blood flow (8).
Cerebral air embolism can be diagnosed if the patient has neurological symptoms after any invasive procedures with cerebral embolic findings and intracranial air on CT or MRI. On brain CT scan, the entrapped air is seen as a circular or serpiginous low attenuation along the path of the blood vessels, and cerebrum shows a lower attenuating lesion than other surrounding tissues by an increased volume of water concentrated in the damaged tissue. Air bubbles rapidly dissolve into the blood or redistribute through the circulation. Therefore, intracranial air densities can be found only in the early stages of disease, and it is difficult to detect the minimal amount of air bubbles on imaging (4). On MRI, GRE images are superior to other diagnostic modalities in the early detection of intracranial air. Multiple small foci of marked hypointensity on GRE suggest cerebral air embolism-particularly, in the patients who have sudden neurological symptoms after the removal of central venous catheter. However, microbleeds in the infarcted area caused by air embolism cannot be excluded (9). In our patient, the GRE image showed multifocal dark signal dots at both frontal lobes (Fig. 1E). Another characteristic finding of cerebral air embolism is the frontal cortical dominancy of air on brain CT and infarct on diffusion-weighted image. Compared to larger bubbles, smaller air bubbles are more resistant to rupture, as the smaller the size of an air bubble, the more the surface tension increases. Thus, air bubbles are more likely to be entrapped in smallsized end arteries at the cortical layers than in larger proximal arteries. Furthermore, the lowest value of flow velocity in the cortical border zone of the anterior and middle cerebral arteries causes the entrapped air bubbles to persistently obstruct blood flow, leading to infarct. Therefore, the frontal cortical area surrounding the border zone of the anterior and middle cerebral arteries should be the most common site of ischemic lesions of cerebral air embolism (10). In our case, DWI showed scattered high signal intensities along both cerebral hemispheres and basal gangli (Fig. 1B, C). Especially, MR finding of confluent high signal intensities at both frontal lobes indicated the presence of the large amount of air.
Although cerebral air embolism rarely occurs when removing catheters (2), there are some preventable procedures. A deep inspiration or upright position that may cause the decrease of central venous pressure should be avoided. Instead, the removal of the catheter should be carried out in a supine position or Trendelenburg position. The patient must hold breath during the procedure. After central venous catheter removal, the exit site must be covered with antibiotic ointment and impermeable dressing for preventing the outside air from entering the venous system through the residual catheter tract. Finally, the patient should be in the supine position for at least half an hour after central venous catheter removal.
In summary, cerebral air embolism is an extremely rare complication of the removal of a central venous catheter. When a central venous catheter is removed, if the patient has any neurological compromise, the physician should consider the possibility of cerebral air embolism with a high level of clinical suspicion and choose appropriate treatment to minimize potential cerebral damage.
Figures and Tables
Fig. 1
Cerebral air embolism following central venous catheter removal in a 67-year old woman with patent foramen ovale.
A. Non-enhanced axial CT image of the brain shows a small linear marked low attenuation lesion (arrow) in the sulcus of the left frontal lobe, suggestive of an air embolism.
B–E. DWI (B, C) demonstrated scattered high signal intensities along both cerebral hemispheres and basal ganglia with marked confluent high signal intensities at both frontal lobes, which were detected as an area of low signal intensity on the ADC map (D), suggesting an acute infarction. GRE image (E) shows multifocal dark signal dots (arrows) in the infarcted area, suggesting the presence of air emboli.
F. TEE shows a flap-like opening between the RA and the LA, suggesting a patent foramen ovale (arrow).
ADC = apparent diffusion coefficient, DWI = diffusion-weighted imaging, GRE = gradient-echo, LA = left atrium, RA = right atrium, TEE = transesophageal echocardiography
References
1. Brockmeyer J, Simot T, Seery J, Johnson E, Armstrong P. Cerebral air embolism following removal of central venous catheter. Mil Med. 2009; 174:878–881.


2. Heckmann JG, Lang CJ, Kindler K, Huk W, Erbguth FJ, Neundörfer B. Neurologic manifestations of cerebral air embolism as a complication of central venous catheterization. Crit Care Med. 2000; 28:1621–1625.


3. Eum DH, Lee SH, Kim HW, Jung MJ, Lee JG. Cerebral air embolism following the removal of a central venous catheter in the absence of intracardiac right-to-left shunting: a case report. Medicine. 2015; 94:e630.
5. Cartin-Ceba R, Swanson KL, Krowka MJ. Pulmonary arteriovenous malformations. Chest. 2013; 144:1033–1044.


6. Lovering AT, Elliott JE, Beasley KM, Laurie SS. Pulmonary pathways and mechanisms regulating transpulmonary shunting into the general circulation: an update. Injury. 2010; 41:Suppl 2. S16–S23.

7. Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006; 34:99–106.


8. Oh JY, Park DW, Hahm CK, Park CK, Lee SR, Lee Y. A cerebral air embolism that developed following defecation in a patient with extensive pulmonary tuberculosis: a case report. J Korean Soc Radiol. 2010; 63:307–310.

9. Jeon SB, Kang DW. Neurological picture. Cerebral air emboli on T2-weighted gradient-echo magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2007; 78:87.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download