Abstract
The authors report a successful outcome after percutaneous transabdominal placement of a self-expandable metallic stent in a patient who had afferent loop syndrome caused by recurrent common bile duct adenocarcinoma. Enhanced abdominal CT showed marked dilation of the afferent loop adjacent to the anterior peritoneum, multiple hepatic metastases and lymph node metastasis around the choledochojejunal anastomosis site without marked dilation of intrahepatic bile ducts. Percutaneous drainage catheter was successfully placed to the dilated afferent loop just below the abdominal wall. Subsequent successful palliation of the afferent loop obstruction was achieved by placing a self-expandable metallic stent via the transabdominal route with the aid of cone-beam CT.
Afferent loop syndrome (ALS) is a well-known complication that occurs after construction of a pancreaticoduodenectomy (PD), Billroth II gastroenterostomy, or other gastrojejunostomies (1). Creation of an anastomosis between the stomach and jejunum leaves an afferent segment which consists of the duodenum and proximal jejunum. ALS results from obstruction of the afferent segment due to a variety of postoperative complications such as adhesion and kinking, and because of recurrent tumors. Traditionally, surgical bypass procedures are considered to be the treatment of choice to resolve obstructive conditions (2). However, most patients with ALS are in poor medical condition and due to the strength of interventional therapies, surgery is rarely a first option.
Percutaneous stent placement has been reported to be a safe and effective treatment method for ALS (345). The two routes for a percutaneous approach are transhepatic and transabdominal. Stent placement using the transhepatic route has been reported by several authors (4678). If the stricture causing ALS is located distal to choledochojejunostomy site with biliary tract dilation, transhepatic route could be considered as a first option for stent placement route. However, if the stricture is located well below the choledochojejunostomy site, it may hard to access by transhepatic approach. Transabdominal approach could be used alternatively, however, only few case reports described use of the transabdominal approach for stent placement in ALS (910). Here, we describe the successful outcome of stent placement using the transabdominal route with the aid of cone-beam CT (CBCT). Our Institutional Review Board approved this report (IRB No. 2019-03-023).
A 72-year-old man who had undergone pylorus-preserving pancreaticoduodenectomy (PPPD) 27 months previously due to common bile duct adenocarcinoma and pancreatic head neuroendocrine tumor was admitted because of high-grade fever and right upper abdominal pain without jaundice. Laboratory data on admission were as follows: white blood cell count, 14920/µL (normal, 4000 to 10000/µL); total bilirubin, 0.89 mg/dL (normal, < 1.0 mg/dL); aspartate aminotransferase, 105 IU/L (normal, < 37 IU/L); alanine aminotransferase, 77 IU/L (normal, < 41 IU/L); alkaline phosphatase, 138 IU/L (normal, 98 to 279 IU/L); γ-glutamul transpeptidase, 40 IU/L (normal, 8 to 45 IU/L); platelet count, 190000/µL (normal, 150000 to 400000/µL); and prothrombin time, 12.1 seconds (normal, 11.0 to 14.2 seconds).
Enhanced abdominal CT obtained after admission showed a markedly distended and kinked afferent loop adjacent to the right anterior peritoneum, multiple masses with low enhancement suggesting hepatic metastasis of adenocarcinoma, and lymph node metastases around the choledochojejunal anastomosis site without dilation of both intrahepatic bile ducts. A transhepatic route was regarded as unsuitable, as there was no sign of jaundice or biliary dilation, and as the kinked afferent loop was unlikely to be resolved by transhepatic biliary stent placement (Fig. 1A, B).
To relieve the obstruction, transabdominal enteric stent placement at the kinked stricture site was planned. As the markedly distended afferent loop was readily accessible via the transabdominal route, ultrasound-guided percutaneous transabdominal drainage was performed using an 8.5 French multi-side hole pigtail catheter (Cook, Bloomington, IL, USA). We did not use gastropexy device during the procedure. Three hundred milliliters of bilious liquid were drained during 6 hours after the procedure, however, amount of daily drainage was not decreased during following 2 days. Stent placement was performed 3 days after percutaneous transabdominal drainage. We infused 30 mL of contrast media through the transabdominal drainage catheter, then used CBCT to visualize the course of the kinked afferent loop. CBCT showed a small amount of contrast media passed through the kinked lesion at posterosuperior aspect of the dilated loop.
We exchanged the pigtail catheter for a 5 French DAV catheter (Cook) over an 0.035-inch, angled, hydrophilic, stiff guidewire (Terumo, Tokyo, Japan). Then, guidewire and catheter were advanced into the duodenum and manipulated across the stricture (Fig. 1C–F). Jejunography confirmed an approximately 4 cm long stricture at the distal afferent loop. After dilation of the transabdominal access tract using 10 French and 12 French dilators, a 20 mm diameter and 8 cm length uncovered self-expandable metallic stent was placed (Choo stent, M.I Tech, Pyeongtaek, Korea). A 12 French multi-side hole pigtail catheter was inserted at the end of the stent placement. Jejunography performed after stent placement showed good passage of contrast media through the stent and efferent loop. There were no procedure-related complications, and there was no leakage of contrast media during procedure. Pigtail catheter was inserted in case early complication such as stent migration or kinking.
The patient's symptoms subsided and his clinical conditions improved 2 days after stent placement, and the pigtail catheter was removed at 3 days after stent placement. Abdominal radiography showed full expansion of the stent 3 days after stent placement. The patient was discharged on day 9 after admission. Enhanced abdominal CT scan performed at 1 month follow-up showed good stent patency with no migration. The patient showed no recurrent ALS symptoms until his death (79 days after stent placement).
Since Caldicott et al. (3) first described stent placement in the ALS via the transhepatic route, there have been a few reports of successful stent placement (45910). There are two main stent placement approaches for treating ALS: the percutaneous and peroral routes. The former includes the percutaneous transhepatic route using the percutaneous transhepatic biliary drainage (PTBD) tract and the percutaneous transabdominal route using enterostomy tubes. The latter includes the peroral endoscopic and peroral fluoroscopic routes. Currently, clear guidelines for selecting the best route have not been established. The approach could be varied according to the type of obstruction, surgery, and associated diseases.
A clear understanding of the level of obstruction and the anatomy of the bypass is essential to select appropriate route. Generally, the percutaneous route provides better access to the lesion in the proximal or mid-portion of the afferent bowel loop. In these cases, distance between the mouth and the obstructive lesion could be too far or too tortuous to use the peroral endoscopic or fluoroscopic approach. In contrast, the peroral approach is a better option for a lesion that is located in the distal portion of the afferent bowel loop, because the distance between the mouth and the lesion is short enough to be able to advance a catheter and guidewire. There are some other factors to consider when choosing access routes other than the obstruction site. Regardless of the obstruction site, if the patient has signs of biliary sepsis due to a dilated afferent loop, PTBD must be done first. Subsequent percutaneous transhepatic stent placement along the PTBD tract is feasible. However, in a patient with massive ascites, PTBD or transabdominal punctures are contraindicated and the peroral approach should be considered.
The percutaneous transhepatic route via the PTBD tract has been widely reported by several authors (4678). Effective palliation has been shown in these reports, but transhepatic access can be challenging in the case of ALS without dilation of the intrahepatic bile ducts. A tortuous and kinked afferent loop stricture at choledochojejunostomy site is another difficult situation that makes transhepatic stent placement less efficient. Stent placement using the percutaneous transabdominal route has been seldom reported (910). The main advantage of this procedure is a short access tract that makes it easy to manipulate a catheter and guidewire, and the puncture site is closest to the stenotic lesion. However, the main concern of a transabdominal approach is leakage of enteric contents into the peritoneal cavity and a collapsed dilated loop. To avoid dislodging the catheter which could lead to leaking of enteric contents, a gastropexy device was used in previous reports (910). This secured safe access, reduced the risk of leaks, peritonitis and allowed for a staged procedure. However, we did not use a gastropexy device because adhesion between anterior peritoneum and afferent loop was expected from serial abdominal CT series.
The recent development of CBCT offers 3-dimensional visualization and more accurate and complex imaging during interventional procedures. In our case, we used CBCT to visualize the course of the contrast medium during the procedure. Structural alteration after PPPD is usually complicated. Especially in ALS patients, tortuosity of the dilated loop makes fluoroscopic procedures difficult. We visualized the 3-dimensional course of the loop using CBCT, thus we were able to complete the procedure easily.
This case report showed that stent placement via a percutaneous transabdominal route with the aid of CBCT was a safe and feasible technique for treating ALS. No complication was noted with this approach even if leaks following the procedure were possible because we did not use a gastropexy device. For patients with ALS who are poor candidates for surgery, especially in cases when there is no marked intrahepatic duct dilation and there is kinked stricture at choledochojejunostomy site, a percutaneous transabdominal approach could be an effective alternative treatment method. Further studies are warranted to determine the safety and effectiveness of this procedure.
Figures and Tables
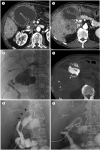 | Fig. 1A 72-year-old man diagnosed with afferent loop syndrome, treated with percutaneous transabdominal metallic stent placement for palliation.
A. Abdominal contrast-enhanced CT on admission shows a markedly dilated afferent loop adjacent to the anterior peritoneum.
B. The afferent loop is kinked at the choledochojejunostomy site (asterisk). Multiple masses with low enhancement suggesting hepatic metastasis of adenocarcinoma, and lymph node metastases around the choledochojejunal anastomosis site are shown without marked dilation of both intrahepatic bile ducts.
C. An 8.5-Fr pigtail catheter is inserted into the afferent loop via the percutaneous transabdominal route. The biliary tree is dilated due to the infused contrast media.
D. Cone-beam CT shows the outflow tract at the posterior aspect of the afferent limb (asterisk).
E. Jejunography confirms an approximately 4-cm-long stricture of the afferent loop (arrows).
F. A 20-mm and 8-cm self-expanding metallic stent was placed across the stenosis via the transabdominal route. A 12-Fr pigtail catheter is placed at the end of stent placement.
|
References
2. Stabile BE, Passaro EJ. Sequels of surgery for peptic ulcer. In : Bockus HL, Berk JE, editors. Bockus gastroenterology. 4th ed. Philadelphia: WB Saunders;1985.
3. Caldicott DG, Ziprin P, Morgan R. Transhepatic insertion of a metallic stent for the relief of malignant afferent loop obstruction. Cardiovasc Intervent Radiol. 2000; 23:138–140.


4. Gwon DI. Percutaneous transhepatic placement of covered, self-expandable nitinol stent for the relief of afferent loop syndrome: report of two cases. J Vasc Interv Radiol. 2007; 18:157–163.

5. Han K, Song HY, Kim JH, Park JH, Nam DH, Ryu MH, et al. Afferent loop syndrome: treatment by means of the placement of dual stents. AJR Am J Roentgenol. 2012; 199:W761–W766.

6. Lee LI, Teplick SK, Haskin PH, Sammon JK, Wolferth C, Amron G. Refractory afferent loop problems: percutaneous transhepatic management of two cases. Radiology. 1987; 165:49–50.


7. McDonald DG, Khalil MF, Vernon JK. Percutaneous transhepatic insertion of a jejunal feeding tube. Radiology. 1983; 148:309–310.


8. Morita S, Takemura T, Matsumoto S, Odani R. Septic shock after percutaneous transhepatic drainage of obstructed afferent loop: case report. Cardiovasc Intervent Radiol. 1989; 12:66–68.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download