Abstract
Spinal cord injury (SCI) has been considered an incurable condition and it often causes devastating sequelae. In terms of the pathophysiology of SCI, reducing secondary damage is the key to its treatment. Various researches and clinical trials have been performed, and some of them showed promising results; however, there is still no gold standard treatment with sufficient evidence. Two therapeutic concepts for SCI are neuroprotective and neuroregenerative strategies. The neuroprotective strategy modulates the pathomechanism of SCI. The purpose of neuroprotective treatment is to minimize secondary damage following direct injury. The aim of neuroregenerative treatment is to enhance the endogenous regeneration process and to alter the intrinsic barrier. With advancement in biotechnology, cell therapy using cell transplantation is currently under investigation. This review discusses the pathophysiology of SCI and introduces the therapeutic candidates that have been developed so far.
Spinal cord injury (SCI) has been considered an incurable condition in spite of enormous advances in medical and surgical treatment. Although an extended understanding of the pathophysiology and accumulation of results from various trials for SCI have opened new possibilities, more objective and convincing evidence is still required for safe and effective clinical application of findings of these trials. This article will discuss the current position of therapeutic trials with a review of the pathophysiology and recent preclinical and clinical trials for SCI.
The overall annual incidence of SCI was estimated at 15–40 cases per million.1) The known causes of SCI are motor vehicle accidents (50%), fall and work-related injuries (30%), violent crimes (11%) and sports-related injuries (9%).2) As these causes are mostly related to physical activity, the second and third decades of life are the predominant ages of the affected patients. A recent study from the US National Spinal Cord Injury Database found that 56% of all SCI cases occur in the cervical spine.3) The second highest incidence of SCI was noted in the patients aged above 50 years. In this group, pre-existing spondylosis is associated with SCI, which results from a relatively low-energy trauma. Besides traumatic SCI, the number of patients with SCI due to other pathologic conditions, such as tumors or demyelinating diseases, is increasing. Metastatic epidural spinal cord compression (MESCC) is one of the causes of nontraumatic SCI. It is estimated that MESCC occurs in 5%–10% of cancer patients and in up to 40% of patients who have pre-existing nonspinal bone metastasis.456)
The pathophysiology of SCI is best described as biphasic, consisting of a primary phase and a secondary phase of injury. The primary injury refers to direct, mechanical injury to the spinal cord. However, in most clinical situations, the secondary mechanism following the primary insult is more important and a therapeutic target for preventing propagation of injury.7)
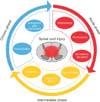
The secondary injury process can be divided into several phases according to the post-injury time and pathomechanism; acute, subacute (or intermediate), and chronic phase (Fig. 1). The acute phase is considered to last 48 hours after the initial physical insult.8910) Vascular disruption, hemorrhage, and the resulting ischemia are major phenomena in this acute phase. Following the disruption of micro-circulation, consequent pathologic changes such as ionic dysregulation, excitotoxicity, excessive production of free radicals and inflammatory response are related to further damage of neurons and glial cells.8111213)
Excitotoxicity is a unique pathologic process that occurs in the central nervous system. It is a result of excessive activation of excitatory neurotransmitters (glutamate, aspartate).14) These over-expressed excitatory amino acids can subsequently cause apoptosis of neurons and glial cells, especially oligodendrocytes.1516)
Free radical-mediated lipid peroxidation results in membrane damage, leading to cell lysis, dysfunction of organelles, and dysregulation of intracellular ionic homeostasis. Reactive oxygen species are one of the major free radicals.1718) Therapeutic trials have shown the neuroprotective effects of antioxidants.
Alteration of the blood-brain barrier (BBB) is noted following injury. Disruption of endothelial cells, loss of function of astrocytes (one of the components for building the BBB), and a direct increase in permeability caused by various inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β are known to be related.19)
Various cellular and hormonal inflammatory mechanisms are related to the secondary mechanism following SCI. Intrinsic microglia, T cells, astrocytes and macrophages are known to participate in major inflammatory reactions. The activation of cellular and hormonal inflammatory mechanisms consequently influences the other secondary mechanisms such as cellular necrosis, apoptosis, production of free radicals, and increased permeability of the BBB directly or indirectly. Recently, dual effects, detrimental and beneficial, of neuroinflammation caused by inflammatory cytokines and macrophages have been reported.20)
Active and/or passive cell death of neurons and glial cells is another mechanism that results in functional neuronal loss. Loss of neurons due to necrosis mainly occurs at all stages of injury.21) However, whether the apoptotic mechanism is related to cellular death is still being investigated. Oligodendrocytes are known to be susceptible to apoptotic cell death.22) The loss of oligodendrocytes results in axonal demyelination, which peaks at 24 hours following injury in the rat.23) Many researchers agree that loss of oligodendrocytes and demyelination are important pathological changes associated with clinical impairments. However, postmortem human studies and a limited number of animal studies have demonstrated that a small number of demyelinated axons or no demyelinated axons were noted following SCI.2425) Further studies are needed for understanding the role of demyelination and remyelination following SCI.
The subacute phase is considered to last until 2 weeks following injury. The peculiar characteristic of this phase is the phagocytic response. The other characteristic of the subacute phase is reactive proliferation of astrocytes. This reactive proliferation results in the formation of an interwovenastrocytic glial scar, which acts as a critical barrier to axonal regeneration, and it is considered as the main cause of limited regeneration after central nervous system (CNS) injury. However, when we consider this process as a protective phenomenon that inhibits the formation of aberrant synapses at the injured site, the reactive proliferation of astrocytes has both detrimental and beneficial effects.262728) Besides glial scar formation, astrocytes also contribute to restoration of microenvironment homeostasis and re-establishment of the integrity of the BBB, which is important for resolution of edema and to limit the infiltration of immune cells.29) At a later stage, astrocytes can aid in regulation by producing a variety of cytokines such as transforming growth factor (TGF)-β, glial cell-derived neurotrophic factor, fibroblast growth factor (FGF)-β and vascular endothelial growth factor (VEGF). Some of these growth factors can facilitate oligodendrocyte precursor cell migration, proliferation and differentiation.
Although there is a debate about the definition of the chronic phase, it is widely accepted that a duration of more than 6 months is the chronic phase. Maturation of the lesion including scar formation and development of a syrinx are considered the characteristics of the chronic phase of SCI. In this chronic stage, therapeutic strategies focus on enhancing regeneration of the damaged axons and remyelination using various pharmacological measures or cell transplantation therapies. Another research target in this stage is to overcome the pathologic barrier, glial scar.
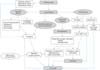
Therapeutic trials to overcome SCI can be categorized into (1) neuroprotective and (2) neuroregenerative approaches. Neuroprotective approach is based on the understanding of the pathomechanism following SCI, and modulation of these pathologic conditions using various pharmacological therapies (Fig. 2). Neuroregenerative trials have been attempted to enhance the endogenous regeneration process, exogenous supplement, and alteration of the intrinsic barrier.
Methylprednisolone (MP) is one of the most commonly used pharmacological agents. The basic background is based on the anti-inflammatory effects of steroids. In an animal study, the antioxidant effect, and reduction of expression of inflammatory cytokines such as TNF-α, IL-1, and IL-6 have been demonstrated.30313233) Based on these experimental results, three major clinical trials have been performed since 1984.343536) Recently, the Congress of Neurological Surgeons released its opinion on the usage of high-dose steroids for treating SCI through a systematic review.37) In this review, it concluded that the beneficial effects of MP administration in the setting of acute SCI are inconsistent with the absence of convincing and significant neurological improvement. Moreover, as harmful side effects of MP in the setting of acute SCI have been reported with high clinical evidence, it denied recommending the use of MP for the treatment of acute SCI.
Gangliosides, sialic acid-containing glycophospholipids, are major components of cell membranes of the mammalian central nervous system. Among them, GM-1 ganglioside has been reported to have neuroprotective and neuroregenerative functions. Induction of neurotrophic factors, anti-excitotoxic effects and promotion of neurite growth in pathologic conditions were also reported.38) Based on these findings, clinical trials of GM-1 in SCI have been performed from the 1990s to the early 2000s.394041) Accelerated recovery of motor function and bowel/bladder function was noted in the first 3 months post-injury in a randomized double-blinded study.41) However, subsequent studies have not been performed to confirm or refute these results in the last decade.
Excitotoxicity, as one of the secondary mechanisms, has been investigated as the target of neuroprotective trials for SCI. Antagonists of ionotropic receptors (N-methyl-D-aspartate [NMDA], α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA]-kainate receptor) have been experimentally investigated for treatment of SCI, and they showed neuroprotective effects on histological and biochemical analyses.12134243) Anti-apoptotic and anti-inflammatory effects and decreased proliferation of astrocytes were reported.12) A phase II clinical trial was also performed using a noncompetitive NMDA receptor antagonist, gacyclidine.44) At the l-month follow-up, clinical results, which were assessed by the American Spinal Injury Association (ASIA) scale, showed an improvement in the studied patients; however, results at 12 months posttreatment failed to show a long-term benefit in this trial. After that, there is a lack of clinical trials and evidence, and experimental trials for preventing excitatory neurotoxicity by using various other agents are still underway.
The receptor for erythropoietin (EPO) is widely observed in the developing and adult brain, and it is upregulated in the condition of trauma. Beneficial effects of peripherally administered EPO on neurogenesis and neuronal differentiation were noted in an animal study.4546) For neuroprotective anti-inflammatory, anti-oxidant effects and induction of neurotrophic factors, there are other proposed mechanisms of neuroprotective effects following SCI.4748) As concerns such as excessive stimulation of hematopoietic activity and the possibility of thrombosis in clinical trials of EPO are suggested, a clinical trial for SCI has not yet been performed.
Intracellular ionic over-influx is related to the excitability of the affected neuron and death of neurons and glial cells. Especially, calcium influx into the cell following SCI is known to result in cell death due to excitotoxicity. Nimodipine, one of the Ca2+ channel blockers, has shown neuroprotective effects in SCI in animal studies.49) But, another report did not demonstrate any superiority compared to the control group at the 1-year follow-up.50)
The physiology of voltage-gated Na+ channels and K+ channels associated with the node of Ranvier in normal myelinated axons is well known. When demyelination occurs due to pathologic conditions, the constraining effect of myelin on K+ channels is lost. Consequently, the action potential progressively declines, resulting in conduction failure and marked slowing of the conduction velocity.
Demyelination of intact and injured axons is a prominent feature of SCI. A double-blind, randomized, placebo-controlled, parallel group phase II clinical trial performed to assess the safety and efficacy of fampridine-sustained-release, a blocker of rapidly inactivating voltage-gated K+ channels, showed a significant improvement in subject global impression scores and spasticity in chronic SCI patients with use of a low dose.51) However, in the patients who received a high dose of fampridine, a higher discontinuation rate was noted due to more frequent adverse side effects such as generalized spasm, hypertonia, insomnia and dizziness compared to the low dose and control groups.
Minocycline is most lipid-soluble of the tetracycline-class antibiotics, showing the greatest penetration into the central nervous system through the BBB. Neuroprotective effects of minocycline have been demonstrated in animal studies of SCI. Its proposed mechanism of neuroprotection includes anti-inflammatory and anti-apoptotic effects and a decrease in the activation of microglia.1352) A recent double-blind, randomized, controlled study performed to assess the safety and dose optimization showed a significant ASIA score improvement in cervical SCI patients at 1 year after injury.53)
Despite promising experimental and clinical results in the 1970s, its efficacy in clinical use has been limited. Studies on hypothermia for treating traumatic SCI became less popular by the 1980s because of potential complications related to systemic hypothermia. Since successful application of hypothermia in an American football player who sustained a cervical SCI is already known, resurgence in the interest in systemic hypothermia has arisen. Neuroprotective effects of systemic and regional hypothermia have been demonstrated.5455) Lately, a technique for rapid and safe induction of hypothermia using an intravascular catheter to reduce systemic complications, such as coagulopathy, sepsis, and cardiac dysrhythmia, was developed and approved by the Food and Drug Administration (FDA). Recent reports on clinical application of modest (33℃) hypothermia showed that 15 of the total 35 patients with cervical SCI improved at least one neurological grade (ISNCSCI) at the last follow-up at 10 months. The overall risk of thromboembolic complications was 14.2%.56) However, because of a lack of sufficient randomized clinical trial data, the Congress of Neurological Surgeons Joint Section on Disorders of the Spine and Peripheral Nerves recently decided that not enough evidence is available to recommend for or against the practice of therapeutic hypothermia as a treatment for SCI.37)
Spontaneous remyelination is known to occur after SCI. Increased remyelination following neural precursor cell transplantation was correlated with improved functional recovery in an animal model of SCI.5758) However, it is possible to achieve axonal conduction ability without remyelination, even if denuded axons persist over a long region of demyelination. For this adaptation, it is known that increased expression of Na+ channels along the length of the demyelinated internodes is required. This upregulation of Na+ channels causes more energy consumption as the influx of sodium is a procedure that requires energy.59) However, as myelination plays essential roles in effective propagation of the action potential and survival of axons and corresponding neurons, remyelination is an attractive therapeutic target in regenerative trials following SCI.
Historically, the following two approaches for improving remyelination have been attempted: cellular transplantation and enhancement of the endogenous repair process. Among various cell sources including Schwann cells, olfactory ensheathing cells, neural progenitor cells and oligodendrocyte progenitor cells (OPCs), OPCs have been widely studied.5960616263) The transplantation of human embryonic stem cell-derived OPCs (hESC-derived OPCs) into rat spinal cord injuries has been found to enhance remyelination and promote improvement in motor function.6465)
Enhancing endogenous repair is another crucial aspect of remyelination. In the pathologic condition caused by SCI, differentiation of OPCs from ependymal stem cells or other neuronal stem cells was also noted besides recruitment of OPCs from surrounding parenchyma.6667) On this background, basic fibroblast growth factor, insulin growth factor 1, and ciliary neurotrophic factor have successfully demonstrated positive effects on proliferation of OPCs.6869)
To date, it is understood that there are two distinct limitations in regeneration after CNS injury: the limited intrinsic regenerative potential and the inhibitory environment of the injured CNS. A comparison of myelin from the CNS and the peripheral nervous system has revealed that CNS white matter is selectively inhibitory for axonal outgrowth. In the late 1980s, Caroni and Schwab7071) demonstrated that oligodendrocyte myelin was a major inhibitor of axonal growth in the CNS. Two inhibitory fractions (35 and 250 kDa) were recognized in these myelin preparations (NI 35 and NI 250, subsequently identified as Nogo), and a monoclonal antibody (termed IN-1) was developed to block these inhibitory factors. The IN-1 antibody also caused a moderate degree of axonal regeneration and functional recovery after SCI.
Chondroitin sulfate proteoglycan (CSPGs) in the injured CNS plays a role in inhibiting axonal regeneration. The degrading enzyme chondroitinase ABC (ChABC) cleaves the inhibitory glycosaminoglycans from the protein core of CSPGs, thereby removing the axonal growth inhibitory properties of intact CSPGs. Local and systemic administration of ChABC caused axonal regeneration and functional recovery in an animal model.7273)
Besides the abovementioned neuroregenerative trials, cell therapies using various cell sources and various manipulations of the transplanted cells have been extensively investigated for treating SCI. However, evidence that supports the differentiation of the transplanted cells into functional neurons is still poor.74)
Neurotrophic support by the transplanted cells is one of the indirect beneficial effects of cell therapy for SCI. Secretion of neurotrophic factors was suggested in various in vitro and in vivo studies. Considering the pathomechanism in the acute phase of SCI, modulation of immune reaction and inflammation has been considered as one of the targets for neuroprotection.
Besides the trials described above, alteration of the microenvironment of the injured spinal cord has been considered as one of the promising targets. Enhancement of survival of the transplanted cells and induction of intrinsic potent stem cells are the goals.
Timing of cell transplantation is one of the most important points that needs to be considered because transplantation in the acute phase of SCI results in a low rate of engraftment by severe inflammatory environment, and transplantation in the chronic phase results in a low rate of engraftment and functional restoration due to a glial scar.74) Therefore, co-transplantation of mesenchymal stem cells with manipulated neural stem cells in the acute phase of SCI has been attempted in order to modulate the acute inflammatory condition and to enhance survival of the transplanted cells.75) To overcome the glial scar, use of chondroitinase ABC (ChABC) for CSPG degradation has also been attempted.
Enhancing the production of endogenous neural/progenitor cells is one of the attractive ways which avoids ethical issues and possible concerns related to cell manipulation. Subventricular zone of the brain and ependymal cells are the sources of cellular recruitment for neural regeneration. Ependymal cells of the spinal cord are descended developmentally from neuroepithelial stem cells located in the ventral neural tube. In lower vertebrates, ependymal cells play an important role in the regeneration observed following spinal cord transection. However, in mammals, who are known to have a lack of regenerative ability, ependymal cells are thought to perform primary functions such as endocrinologic or protective functions instead of regenerative functions. Recent studies show that ependymal cells undergo reactive proliferation, and express neural stem cell properties following SCI.767778) Further work is still needed to determine the exact mechanism and its therapeutic possibility.
It has been universally accepted that SCI is incurable and results in permanent disabilities. However, various pharmacological agents and hypothermia have shown some therapeutic possibility for the treatment of SCI. MP is one of the most popular neuroprotective agents, but its harmful effects always cause hesitation about its use. Hypothermia could also be an effective neuroprotective treatment for SCI, even though there is still a lack of sufficient evidence. Another treatment strategy for SCI is the neuroregenerative approach. Compared to neuroprotective treatment with unavoidable side effects, enhancing endogenous regenerative capability could be a more effective strategy. Recently, cell therapy for neurotrophic support has emerged as the treatment for SCI.
Various clinical trials have shown promising results; however, there is no gold standard yet. In the future, a novel treatment protocol using both neuroprotective and neuroregenerative approaches should be developed.
Figures and Tables
ACKNOWLEDGEMENTS
This article was supported by a grant from Catholic Institute of Cell Therapy in 2016.
References
1. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001; 26:24 Suppl. S2–S12.

2. Ho CH, Wuermser LA, Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC. Spinal cord injury medicine: 1. epidemiology and classification. Arch Phys Med Rehabil. 2007; 88:3 Suppl 1. S49–S54.

3. Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005; 87(2):260–267.

4. Barron KD, Hirano A, Araki S, Terry RD. Experiences with metastatic neoplasms involving the spinal cord. Neurology. 1959; 9(2):91–106.

5. Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Williston Park). 2000; 14(7):1013–1024.
6. Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine (Phila Pa 1976). 1985; 10(1):19–20.

7. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008; 25(5):E2.

8. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004; 4(4):451–464.

9. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991; 75(1):15–26.

10. Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997; 86(3):483–492.

11. Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979; 206(4419):700–702.

12. Ha KY, Carragee E, Cheng I, Kwon SE, Kim YH. Pregabalin as a neuroprotector after spinal cord injury in rats: biochemical analysis and effect on glial cells. J Korean Med Sci. 2011; 26(3):404–411.

13. Ha KY, Kim YH, Rhyu KW, Kwon SE. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J. 2008; 17(6):864–872.

14. Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000; 20(3):1190–1198.

15. Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999; 19(14):RC16.

16. Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004; 21(6):754–774.

17. Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008; 209(2):378–388.

18. Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007; 100(3):639–649.

19. Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007; 500(2):267–285.

20. Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006; 129(Pt 12):3249–3269.

21. Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002; 137:37–47.
22. Stys PK, Lipton SA. White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol Sci. 2007; 28(11):561–566.

23. Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005; 486(4):373–383.

24. Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004; 42(10):549–563.

25. Lasiene J, Shupe L, Perlmutter S, Horner P. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci. 2008; 28(15):3887–3896.

26. Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000; 32(1):1–14.

27. Williams A, Piaton G, Lubetzki C. Astrocytes: friends or foes in multiple sclerosis? Glia. 2007; 55(13):1300–1312.
28. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004; 24(9):2143–2155.

29. Herrmann JE, Imura T, Song B, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008; 28(28):7231–7243.

30. Pan JZ, Ni L, Sodhi A, Aguanno A, Young W, Hart RP. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res. 2002; 68(3):315–322.

31. Black P, Markowitz RS. Experimental spinal cord injury in monkeys: comparison of steroids and local hypothermia. Surg Forum. 1971; 22:409–411.
32. Campbell JB, DeCrescito V, Tomasula JJ, Demopoulos HB, Flamm ES, Ransohoff J. Experimental treatment of spinal cord contusion in the cat. Surg Neurol. 1973; 1(2):102–106.
33. Green BA, Kahn T, Klose KJ. A comparative study of steroid therapy in acute experimental spinal cord injury. Surg Neurol. 1980; 13(2):91–97.
34. Bracken MB, Shepard MJ, Hellenbrand KG, et al. Methylprednisolone and neurological function 1 year after spinal cord injury: results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985; 63(5):704–713.

35. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405–1411.

36. Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial: National Acute Spinal Cord Injury Study. JAMA. 1997; 277(20):1597–1604.

37. Hurlbert RJ, Hadley MN, Walters BC. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013; 72:Suppl 2. 93–105.

38. Bose B, Osterholm JL, Kalia M. Ganglioside-induced regeneration and reestablishment of axonal continuity in spinal cord-transected rats. Neurosci Lett. 1986; 63(2):165–169.

39. Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury: a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991; 324(26):1829–1838.

40. Geisler FH. GM-1 ganglioside and motor recovery following human spinal cord injury. J Emerg Med. 1993; 11:Suppl 1. 49–55.
41. Geisler FH, Coleman WP, Grieco G, Poonian D. Sygen Study Group. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976). 2001; 26:24 Suppl. S87–S98.

42. Wrathall JR, Teng YD, Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D-aspartate receptors. Exp Neurol. 1996; 137(1):119–126.

43. Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol. 1997; 145(2 Pt 1):565–573.

44. Tadie M, d'Arbigny P, Mathe JF, et al. Acute spinal cord injury: early care and treatment in a multicenter study with gacyclidine. Soc Neurosci Abstr. 1999; 25(1-2):1090.
45. Boran BO, Colak A, Kutlay M. Erythropoietin enhances neurological recovery after experimental spinal cord injury. Restor Neurol Neurosci. 2005; 23(5-6):341–345.
46. King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007; 26(1):90–100.

47. Yazihan N, Uzuner K, Salman B, Vural M, Koken T, Arslantas A. Erythropoietin improves oxidative stress following spinal cord trauma in rats. Injury. 2008; 39(12):1408–1413.

48. Okutan O, Solaroglu I, Beskonakli E, Taskin Y. Recombinant human erythropoietin decreases myeloperoxidase and caspase-3 activity and improves early functional results after spinal cord injury in rats. J Clin Neurosci. 2007; 14(4):364–368.

49. Fehlings MG, Tator CH, Linden RD. The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J Neurosurg. 1989; 71(3):403–416.

50. Petitjean ME, Pointillart V, Dixmerias F, et al. Medical treatment of spinal cord injury in the acute stage. Ann Fr Anesth Reanim. 1998; 17(2):114–122.
51. Cardenas DD, Ditunno J, Graziani V, et al. Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord. 2007; 45(2):158–168.

52. Yune TY, Lee JY, Jung GY, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007; 27(29):7751–7761.

53. Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012; 135(Pt 4):1224–1236.

54. Ok JH, Kim YH, Ha KY. Neuroprotective effects of hypothermia after spinal cord injury in rats: comparative study between epidural hypothermia and systemic hypothermia. Spine (Phila Pa 1976). 2012; 37(25):E1551–E1559.
55. Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976). 2008; 33(19):2059–2065.

56. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013; 51(5):395–400.

57. Duncan ID, Brower A, Kondo Y, Curlee JF Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A. 2009; 106(16):6832–6836.

58. Liebetanz D, Merkler D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol. 2006; 202(1):217–224.

59. Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006; 7(12):932–941.

60. Bretzner F, Plemel JR, Liu J, Richter M, Roskams AJ, Tetzlaff W. Combination of olfactory ensheathing cells with local versus systemic cAMP treatment after a cervical rubrospinal tract injury. J Neurosci Res. 2010; 88(13):2833–2846.

61. Ramer LM, Richter MW, Roskams AJ, Tetzlaff W, Ramer MS. Peripherally-derived olfactory ensheathing cells do not promote primary afferent regeneration following dorsal root injury. Glia. 2004; 47(2):189–206.

62. Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004; 473(1):1–15.

63. Biernaskie J, Sparling JS, Liu J, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007; 27(36):9545–9559.

64. Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005; 49(3):385–396.

65. Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005; 25(19):4694–4705.

66. Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006; 498(4):525–538.

67. Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009; 57(3):270–285.

68. Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993; 118(1):283–295.

69. Tripathi RB, McTigue DM. Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. J Comp Neurol. 2008; 510(2):129–144.

70. Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988; 1(1):85–96.

71. Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988; 106(4):1281–1288.

72. Barritt AW, Davies M, Marchand F, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006; 26(42):10856–10867.

73. Shields LB, Zhang YP, Burke DA, Gray R, Shields CB. Benefit of chondroitinase ABC on sensory axon regeneration in a laceration model of spinal cord injury in the rat. Surg Neurol. 2008; 69(6):568–577.

74. Kim JW, Ha KY, Molon JN, Kim YH. Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine (Phila Pa 1976). 2013; 38(17):E1065–E1074.
75. Kim DH, Yoo KH, Yim YS, et al. Cotransplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci. 2006; 21(6):1000–1004.

76. Panayiotou E, Malas S. Adult spinal cord ependymal layer: a promising pool of quiescent stem cells to treat spinal cord injury. Front Physiol. 2013; 4:340.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download