Abstract
Purpose
This study was designed to investigate the relationship between molecular subtype and locoregional recurrence (LRR) in patients with early-stage breast cancer with 1–3 positive axillary lymph nodes (ALNs) and improve the individualized indications for postmastectomy radiotherapy (PMRT).
Methods
The records of 701 patients with pT1-2N1M0 breast cancer who did not undergo PMRT were retrospectively analyzed. Tumors were subclassified as follows: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and basal-like subtypes. Multivariate Cox analysis was used to determine the risk of LRR associated with the different subtypes and to adjust for clinicopathologic factors.
Results
Luminal A, luminal B, HER2-enriched, and basal-like subtypes accounted for 51.2%, 28.0%, 8.1%, and 12.7% of cases, respectively. The median follow-up duration was 67 months (range, 9–156 months). Univariate analysis revealed that, compared with the luminal A subtype, the HER2-enriched and basal-like subtypes were associated with significantly higher 5-year LRR rates (5.6% vs. 21.6% and vs.15.7% respectively; p=0.002 each), lower 5-year LRR-free survival (LRFS) rates (90.6% vs. 73.8% and 78.5%, respectively; p=0.001 each), and poorer 5-year breast cancer-specific survival (BCSS) rates (93.7% vs. 82.2% [p=0.002] and 84.9% [p=0.001], respectively). Multivariate analysis revealed that the HER2-enriched and basal-like subtypes, age ≤35 years, a medial tumor, and pT2 stage were poor prognostic factors for LRR and LRFS; furthermore, 2 to 3 positive ALNs represented an independent prognostic factor affecting LRR. The 10-year LRR rates of patients with 0, 1, 2, 3, and 4 risk factors were 1.0%, 6.9%, 14.3%, 30.4%, and 54.3%, respectively (p<0.001); the 10-year BCSS rates were 86.6%, 88.5%, 84.4%, 79.7%, and 38.8%, respectively (p<0.001).
Radiotherapy is an important adjuvant treatment for patients with breast cancer. The general consensus is that postmastectomy radiotherapy (PMRT) is indicated for patients with a high risk of recurrence, such as patients with T3 tumors with positive axillary lymph nodes (ALNs) and patients with ≥4 positive ALNs. However, the use of PMRT in patients with early-stage breast cancer with 1–3 positive ALNs (pT1- 2N1M0) is somewhat controversial. Recently, in a subgroup analysis, two important studies showed that patients with 1–3 positive ALNs who underwent regional nodal irradiation did not have a clear survival benefit [12]. In contrast, a metaanalysis of 22 randomized trials carried out by the Early Breast Cancer Trialists' Collaborative Group showed that patients with 1–3 positive ALNs benefited from PMRT, even in combination with systemic therapy [3]. Since 2007, the National Comprehensive Cancer Network's clinical practice guidelines for breast cancer have strongly recommended that PMRT be considered for patients with early-stage breast cancer with 1–3 positive ALNs [4]. Therefore, it remains unclear whether this subgroup of patients should receive PMRT.
Some authors reported that subgroups with a comparatively high risk of locoregional failure after mastectomy exist among patients with early-stage breast cancer and 1–3 positive ALNs [56]. Various clinicopathologic features, such as age, primary tumor size and location, number and proportion of positive ALNs, and lymphovascular invasion, have been examined to determine if they are associated with an increased risk of locoregional failure [78]. However, breast cancer is known to be a highly heterogeneous tumor, and treatment options are based on not only clinicopathologic criteria but also the intrinsic biologic features of the tumor. Recent gene expression profiling studies have shown that breast cancer consists of several biologically distinct molecular subtypes that are associated with different clinical characteristics and outcomes [9]. In addition, previous studies have demonstrated that the molecular subtypes of breast cancer, which reflect the intrinsic nature of the tumor cells, can provide more prognostic information to facilitate treatment decisions [1011].
In this study, we retrospectively analyzed the association between the molecular subtypes of breast cancer and locoregional recurrence (LRR) in a cohort of patients with earlystage breast cancer. We explored the use of molecular subtyping in combination with clinicopathologic features to improve individualized indications for PMRT.
The cases of breast cancer patients who were diagnosed and treated at two institutions between September 1998 and December 2010 were retrospectively reviewed. This study was approved by the respective institutional review boards (approval number: YP2012-03-15).
The included cases were selected according to the following criteria: (1) female patients with unilateral breast lesions; (2) radical mastectomy or modified radical mastectomy and no preoperative anti-tumor therapy or PMRT; (3) pathological stage of pT1-2N1M0 according to the 2010 American Joint Committee on Cancer (AJCC) breast cancer staging system; (4) complete pathological/immunohistochemical examination or fluorescence in situ hybridization (FISH) and treatment records; and (5) no previous history of malignancy. All patients provided written consent for storage and research use of their medical information.
In total, 701 patients were enrolled in this study; their clinical data are shown in Table 1. The median patient age at diagnosis was 49 years (range, 23–82 years). Left-sided and rightsided breast cancers were found in 354 patients and 347 patients, respectively. One hundred and four patients underwent standard radical mastectomy (Halsted operation), while 597 patients underwent a modified radical mastectomy (Auchincloss' or Patey's operation). Tumor pathology included infiltrating ductal carcinoma in 692 cases, infiltrating lobular carcinoma in three cases, medullary carcinoma in four cases, and simple carcinoma in two cases. Angiovascular invasion was found in 21 cases.
Menopause was defined as the permanent cessation of menses. Patients were considered postmenopausal at the date of diagnosis when they met any of the following criteria: (1) prior bilateral oophorectomy; (2) age ≥60 years; and (3) age <60 years, but amenorrheic for ≥12 months without any other obvious pathological or physiological cause [4]. Perimenopause refers to the period immediately before menopause, which is characterized by irregular menstrual cycles, and to the first year after menopause [12].
Breast quadrants are defined by 12, 3, 6, and 9 o'clock "lines" that radiate perpendicularly from the nipple. A central tumor was defined as a lesion centrally located in the nipple-areola complex. A medial tumor was defined as a lesion centrally located on the sternum side of the 12 and 6 o'clock "lines," including the upper medial quadrant and lower medial quadrant; a lateral tumor was defined as a lesion centrally located on the axillary side of the 12 and 6 o'clock "lines," including the upper lateral quadrant and lower lateral quadrant of the breast outside the nipple-areola complex. The tumor locations of all patients were determined based on a physical exam and on the results of the breast imaging procedures, which included digital mammography, ultrasound, and magnetic resonance imaging.
Estrogen receptor (ER) and progesterone receptor (PR) status was semiquantitatively and quantitatively determined using immunohistochemistry (IHC). For the semiquantitative measurement, ER- and PR-positivity was defined as an ER/PR Histo (H)-score ≥10%. The ER/PR H-scores were grouped as follows: <10%, negative (-); 10% to 25%, weakly positive (1+); 26% to 50%, positive (2+); and >50%, strongly positive (3+). The ER/PR H-score was calculated as a weighted sum of the intensity of the tumor cell nuclei expressing the hormone receptor markers as follows: ER/PR H-score=(percentage of positively stained tumor cell nuclei in the weak intensity category [1+]×1)+(percentage of positively stained tumor cell nuclei in the intermediate intensity category [2+]×2)+(percentage of positively stained tumor cell nuclei in the strong intensity category [3+]×3). A minimum of 100 tumor cells were scored, and the percentage of tumor cell nuclei in each category was recorded [13]. For the quantitative measurement, ER- and PR-positivity was defined as ≥1% of tumor cells showing positive nuclear staining of any intensity; negative staining was reported if the percentage of tumor cells showing staining of any intensity was <1%. A minimum of 100 tumor cells were scored, and the percentage of tumor cell nuclei in each category was recorded [14]. Semiquantitative and quantitative measures were used in 311 and 390 cases, respectively, and 555 cases were ER- and/or PR-positive.
Human epidermal growth factor receptor 2 (HER2) status in IHC and FISH examinations was based on the Breast Cancer HER2 Testing Guidelines [15]. Tumors were considered HER2-positive if they had a score of 3+ or 2+ on IHC and this score was confirmed with FISH. HER2-positivity was determined in 120 cases.
Ki-67-positive cells typically displayed tan-yellow nuclear staining, and rarely exhibited weak cytoplasmic staining. According to the proportion of positive cells in the selected highresolution field, the results were grouped as follows: <10%, negative (-); 10% to 25%, weakly positive (1+); 26% to 50%, positive (2+); and >50%, strongly positive (3+). Among cases with a Ki-67 status of 1+, the positive indices were re-evaluated: <14% were identified in 62 cases, and ≥14% were identified in 127 cases.
For molecular typing, patients were categorized into four subtypes mainly based on the IHC indicators of their primary tumor: luminal A (ER+ or PR+, HER2-, and Ki-67 <14%) in 359 cases; luminal B ([ER+ or PR+, HER2-, and Ki-67 ≥14%] or [ER+ or PR+ and HER2+]) in 196 cases; HER2-enriched (ER- and PR- and HER2+) in 57 cases; and basal-like (ER- and PR- and HER2-) in 89 cases.
All patients who received adjuvant radiotherapy were excluded from this study. In total, 660 patients received chemotherapy, with a median of six cycles (range, 1–8 cycles). Of 479 patients who received endocrine therapy, 180 were treated for <3 years, 203 were treated for 3 to 5 years, and 96 were treated for >5 years. Additionally, 14 patients with HER2-positive tumors underwent targeted therapy with trastuzumab.
In addition to in-person patient consultations following surgery, follow-ups were also accomplished through phone calls and correspondences. Local and regional lymph node recurrence, distant metastasis, and survival status were recorded. The primary endpoints were LRR and LRR-free survival (LRFS). The secondary endpoint was breast cancer-specific survival (BCSS). LRR was defined as any recurrence within the ipsilateral chest wall or ipsilateral regional lymph nodes, including the axillary, supraclavicular, and internal mammary nodes, with the same histopathologic features as the primary tumor as confirmed by pathological biopsy, regardless of whether distant metastases were present. LRFS was calculated from the date of diagnosis to the date of LRR, death due to any cause, or the last follow-up. BCSS was calculated from the date of diagnosis to the date of death from breast cancer or the last follow-up.
The SPSS statistical software package version 16.0 (SPSS Inc., Chicago, USA) was used to establish the database. The Kaplan-Meier method was adapted for the univariate survival analysis. The log-rank test was used to compare the survival distributions. A Cox proportional hazards model was used for the multivariate analysis. Pearson chi-square test was used to compare the distributions of baseline characteristics among the four subtypes. All statistical tests were two-sided, and p<0.05 was considered statistically significant.
The frequencies of the different breast cancer subtypes were as follows: luminal A, 51.2%; luminal B, 28.0%; HER2-enriched, 8.1%; and basal-like, 12.7%. Compared with the luminal A subtype, luminal B, HER2-enriched, and basal-like subtypes were more likely to be histological grade III (11.4% vs. 25.5%, 19.3%, and 21.3%, respectively, p<0.001) (Table 2).
The follow-up deadline was March 31, 2015. The follow-up rate was 97.1%. The median follow-up duration was 67 months (range, 9–156 months). For the entire study population, the 5-year and 10-year LRR rates were 9.1% and 13.5%, respectively; the 5-year and 10-year LRFS rates were 86.3% and 77.5%, respectively; and the 5-year and 10-year BCSS rates were 91.2% and 84.0%, respectively.
There were 77 deaths, including 66 patients who died of breast cancer, 10 patients who died of a disease other than breast cancer, and one patient who died of a secondary tumor. Of 124 patients who developed recurrence and metastasis, 62 developed LRR, with a median LRR time of 55 months after surgery (range, 4–156 months). Additionally, 31 of the 62 LRR cases had distant metastasis. The sites of LRR sites were as follows: 25 (40.3%) in the chest wall, 34 (54.8%) in the supraclavicular area, 10 (16.1%) in the axilla, and seven (11.3%) in the internal mammary area. Twelve patients experienced recurrence at ≥2 sites. Furthermore, the proportion of the internal mammary area affected by LRR was higher in patients with tumors of the medial breast than in patients with tumors of the central/lateral breast (18.5% [5/27] vs. 5.7% [2/35], p=0.161).
The patients' clinicopathologic characteristics are shown in Table 1. The results of the univariate analysis indicated that the differences in the 5-year LRR and LRFS rates between the luminal A subtype and the luminal B subtype were not statistically significant (p=0.137); however, the HER2-enriched and basal-like subtypes were associated with an increased risk of LRR (p=0.002 each), lower LRFS (p=0.001 each), and lower BCSS (p=0.002 and p=0.001, respectively) (Table 3, Figure 1).
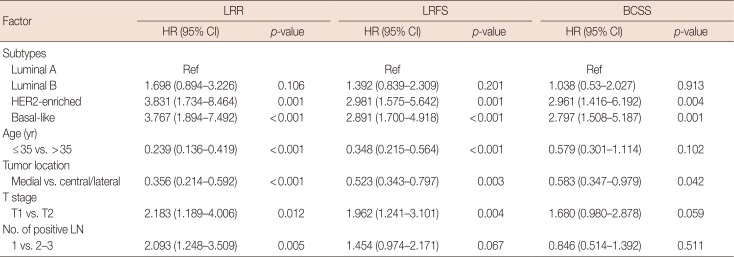
Factors with p<0.1 in the univariate analysis were included in the multivariate analysis. The results showed that the HER2-enriched and basal-like subtypes, age ≤35 years, a medial tumor location, and pT2 stage were independent prognostic factors that impacted LRR and LRFS; furthermore, the presence of 2-3 positive ALNs represented an independent prognostic factor of LRR. The HER2-enriched and basal-like subtypes, and a medial tumor location were independent prognostic factors that affected BCSS (Table 4).
The data were grouped according to the prognostic factors mentioned above. The results suggested that the 5-year LRR rates for patients with 0 (14.4%), 1 (30.8%), 2 (36.5%), 3 (16.0%), and 4 (2.3%) risk factors were 1.0%, 4.7%, 7.1%, 23%, and 54.3%, respectively, and that the 10-year LRR rates were 1.0%, 6.9%, 14.3%, 30.4%, and 54.3%, respectively (p<0.001). Additionally, the 5-year BCSS rates for patients with 0, 1, 2, 3, and 4 risk factors were 94.4%, 92.2%, 92.3%, 90.2%, and 58.2%, respectively, and the 10-year BCSS rates were 86.6%, 88.5%, 84.4%, 79.7%, and 38.8%, respectively (p<0.001) (Figure 2).
With the development of the genotyping concept, molecular typing has become the standard practice for the guidance of chemotherapy and endocrine therapy in patients with breast cancer. However, only a few studies have evaluated the utility of molecular typing in guiding decisions regarding PMRT in patients with early-stage breast cancer.
Biomarkers associated with breast cancer prognosis include ER, PR, HER2, and Ki-67. Previous studies have evaluated the prognostic ability of single molecular markers, but the results have been inconsistent; a single molecular marker may not accurately reflect the intrinsic biological differences across tumors [16]. Studies have also examined whether approximate molecular typing at the protein level based on IHC biomarkers can more accurately predict the biological and phenotypic differences in early-stage breast cancer. Wo et al. [17] analyzed tumors from 1,000 patients with early-stage breast cancer who were treated with breast-conserving surgery (BCS), and found that both the HER2-enriched (p=0.010) and basal-like (p=0.080) subtypes were associated with higher rates of isolated regional nodal failure, compared with the luminal A subtype. Nguyen et al. [10] examined the approximate molecular subtypes of 793 patients who underwent BCS and radiation according to IHC analysis of ER, PR, and HER2 levels; in this study, multivariable analyses showed that the LRR risk in patients with HER2-enriched or basal-like subtypes was 9.2- and 7.1-fold greater, respectively, than that in patients with the luminal A subtype. Furthermore, Voduc et al. [18] analyzed 2,985 tumors that were treated with either BCS or mastectomy between 1986 and 1992 and found that molecular typing using six IHC indicators (ER, PR, HER2, Ki-67, CK5/6, and EGFR) demonstrated good predictive value in identifying patients with a higher risk of LRR. In a subgroup analysis of patients with grade 3 T1-2N0-1 breast cancer, the local relapse rate after mastectomy was 22% in patients with luminal B tumors, compared with only 8% in patients with luminal A tumors; in patients with grade 3 T2N0-1 breast cancer who did not undergo radiotherapy treatment of their regional lymph nodes, the regional relapse rate was 20% in patients with basal- like tumors, compared with 8% for those with luminal A tumors. Kim et al. [19] reported that in patients with stage I breast cancer, those with triple-negative breast cancer had poorer 10-year relapse-free survival (75.6% vs. 87.5%, p= 0.004) and overall survival (OS) (83.0% vs. 91.4%, p=0.002) rates than the patients with non-triple-negative breast cancer. These findings suggest that these patients may benefit from additional adjuvant treatment. In contrast, in a large cohort study of 884 patients with T1-2 breast cancer and 1–3 positive ALNs, Moo et al. [20] found that molecular subtype was not associated with LRR, but that it was associated with LRFS and OS. The authors also found that the basal and HER2 subtypes had the lowest LRFS (p<0.001) and OS (p<0.001) rates. They concluded that molecular subtyping may not be useful in the identification of patients who would benefit from PMRT among the subgroup of patients included in their study. In our study, the univariate analysis showed that, compared with the luminal A subtype, the HER2-enriched and basal-like subtypes were associated with significantly higher 5-year LRR rates (5.6% vs. 21.6% and 15.7%, respectively; p=0.002 each) and lower 5-year LRFS rates (90.6% vs. 73.8% and 78.5%, respectively; p=0.001 each). Multivariate analysis revealed that the risk of LRR associated with the HER2-enriched and basallike subtypes was 3.83- and 3.77-fold greater, respectively, than that associated with the luminal A subtype. Furthermore, our study found that, compared with the luminal A subtype, the HER2-enriched subtype and the basal-like subtype conveyed worse 5-year BCSS rates (93.7% vs. 82.2% [p=0.002] and 84.9% [p=0.001], respectively); additionally, they were more likely to be of histological grade 3 (11.4% vs. 19.3% and 21.3%, respectively; p<0.001 each).
The multivariate analysis also showed that age ≤35 years, a medial tumor, pT2 stage, and the presence of 2-3 positive ALNs were risk factors for LRR. Younger patients have a greater risk of relapse than older patients. Voogd et al. [21] found that the LRR risk in a breast cancer patient ≤35 years of age was 9.24 times greater than that in a patient ≥60 years of age. Su et al. [22] also reported that patient age <40 years (p=0.004) was associated with a higher risk of LRR in patients with T1-2 and N1 cancer. The high risk of recurrence in younger patients with breast cancer may be due to the more aggressive tumor behavior that is often observed in these patients, which is indicated by poor differentiation grade, a higher proportion of cells in S phase, an increased risk of lymphovascular invasion, and a high rate of ER- tumors [23].
In previous studies, tumor location was an inconsistent prognostic factor for LRR. Several studies have reported no correlation between a medial location and LRR and survival in patients with early-stage breast cancer [2425]. However, Gaffney et al. [26] showed that an inner quadrant tumor location was associated with poorer BCSS and OS compared with an outer quadrant location. Shen et al. [27] reported that a medial tumor location was associated with a higher risk of ALN recurrence. Truong et al. [5] demonstrated that in a subgroup of patient ≥45 years of age with a nodal ratio >0.25 and ER negative status, medial tumor location was associated with an LRR risk of 40% to 50%. Our study also found that medial tumor location was a poor individual indicator of LRR, LRFS, and BCSS. This finding may be because medial tumors often drain into the internal mammary nodes (18.5% vs. 5.7% for central/lateral tumors) and then to the supraclavicular lymph nodes.
Regarding T stage and the number of involved ALNs, Cheng et al. [24] reported that the 4-year LRR rates in patients with stage T2 and T1 early breast cancer were 22.2% and 6.9%, respectively, suggesting poor local control in patients with stage T2 breast cancer. Truong et al. [5] found that the risk of local recurrence was >20% in patients with T2 breast cancer who did not receive PMRT. Sharma et al. [6] also found that the 10-year LRR of patients with stage T2 breast cancer was higher than that of patients with stage T1 cancer (p=0.020). In addition, several reports demonstrated that patients with 2–3 positive nodes had a higher risk of supraclavicular fossa recurrence than those with 1 positive node, suggesting that supraclavicular fossa radiotherapy should be considered in the subset of patients with 2–3 positive nodes [2829].
For patients whose 10-year LRR risk is <10%, PMRT is unnecessary due to the low incidence of LRR; PMRT would not provide any additional benefit. However, if the 10-year LRR risk is ≥25%, PMRT may help to improve local control rates and, hence, provide a survival benefit. When the risk of LRR is between 10% and 25%, the pros and cons of PMRT should be weighed before a treatment decision is made [530]. Previous studies have shown that a 20% reduction in the absolute LRR risk would improve the BCSS rate by approximately 4%-5% [30]. In this study, the 10-year LRR rate of the entire study population was 13.5%. We examined whether it was possible to select subgroups with high-risk factors for recurrence according to the results of the multivariate analysis for PMRT. The results showed that the 10-year LRR rates of patients with 0, 1, 2, 3, and 4 LRR risk factors were 1.0%, 6.9%, 14.3%, 30.4%, and 54.3%, respectively, and that the 10-year BCSS rates were 86.6%, 88.5%, 84.4%, 79.7%, and 38.8%, correspondingly; thus, we propose that patients with ≥3 risk factors should receive PMRT.
We acknowledge several limitations of this study. First, this study was a retrospective analysis with a relatively small sample size; therefore, there may have been an inherent selection bias. Second, we performed subtype classifications based on IHC surrogates for genotype-based breast cancer molecular subtypes. Some HER2 data were obtained through IHC testing because the FISH test was not always available. This testing method might have resulted in misclassification of some cases in which the HER2 IHC score was 2+. Third, although our results showed that the HER2-enriched subtype was an independent prognostic factor for LRR, LRFS, and BCSS, most patients did not undergo trastuzumab treatment. In addition, not all patients (479/555, 86.3%) with positive hormone receptor status received endocrine therapy, and only 96 patients were treated for >5 years, which may have influenced the results to a certain extent. Fourth, although the majority of LRR events occur within the first 5 years after treatment, in some cases, the follow-up duration in our study was not long enough to fully evaluate the rate of LRR. Despite these limitations, we believe that our findings will help identify the highrisk subgroup of patients with N1 early-stage breast cancer who may benefit from PMRT.
In conclusion, molecular subtyping using four IHC biomarkers could provide clinically useful information regarding tumor biology and clinical behaviors and identify patients with N1 early-stage breast cancer who are at increased risk of LRR. HER2-enriched and basal-like subtypes, age ≤35 years, a medial tumor, pT2 stage, and the presence of 2–3 positive ALNs were identified as independent poor prognostic factors that affected LRR in this study of patients who did not receive PMRT. For patients with ≥3 risk factors, PMRT to the chest wall and supraclavicular area should be recommended.
Notes
References
1. Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015; 373:307–316. PMID: 26200977.

2. Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015; 373:317–327. PMID: 26200978.

3. EBCTCG (Early Breast Cancer Trialists' Collaborative Group). McGale P, Taylor C, Correa C, Cutter D, Duane F. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014; 383:2127–2135. PMID: 24656685.
4. Clinical practice guidelines in oncology - breast cancer V.2. 2015. National Comprehensive Cancer Network. Accessed Mar 11th, 2015. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
5. Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005; 61:1337–1347. PMID: 15817335.

6. Sharma R, Bedrosian I, Lucci A, Hwang RF, Rourke LL, Qiao W, et al. Present-day locoregional control in patients with T1 or T2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol. 2010; 17:2899–2908. PMID: 20443145.

7. Hamamoto Y, Ohsumi S, Aogi K, Shinohara S, Nakajima N, Kataoka M, et al. Are there high-risk subgroups for isolated locoregional failure in patients who had T1/2 breast cancer with one to three positive lymph nodes and received mastectomy without radiotherapy? Breast Cancer. 2014; 21:177–182. PMID: 22569681.

8. Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer. 2011; 14:198–203. PMID: 22031801.

9. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70. PMID: 23000897.
10. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008; 26:2373–2378. PMID: 18413639.

11. Park YH, Lee SJ, Cho EY, Choi YL, Lee JE, Nam SJ, et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2011; 22:1554–1560. PMID: 21242587.

12. Research on the menopause in the 1990s: report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996; 866:1–107. PMID: 8942292.
13. McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW, et al. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990; 50:3545–3550. PMID: 2187598.
14. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795. PMID: 20404251.

15. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/ College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014; 138:241–256. PMID: 24099077.
16. Albert JM, Gonzalez-Angulo AM, Guray M, Sahin A, Strom EA, Tereffe W, et al. Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,bN0 breast cancer. Int J Radiat Oncol Biol Phys. 2010; 77:1296–1302. PMID: 20472353.

17. Wo JY, Taghian AG, Nguyen PL, Raad RA, Sreedhara M, Bellon JR, et al. The association between biological subtype and isolated regional nodal failure after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2010; 77:188–196. PMID: 20171798.

18. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–1691. PMID: 20194857.

19. Kim JE, Ahn HJ, Ahn JH, Yoon DH, Kim SB, Jung KH, et al. Impact of triple-negative breast cancer phenotype on prognosis in patients with stage I breast cancer. J Breast Cancer. 2012; 15:197–202. PMID: 22807937.

20. Moo TA, McMillan R, Lee M, Stempel M, Ho A, Patil S, et al. Impact of molecular subtype on locoregional recurrence in mastectomy patients with T1-T2 breast cancer and 1-3 positive lymph nodes. Ann Surg Oncol. 2014; 21:1569–1574. PMID: 24488216.

21. Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Breast Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer: differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001; 19:1688–1697. PMID: 11250998.
22. Su YL, Li SH, Chen YY, Chen HC, Tang Y, Huang CH, et al. Postmastectomy radiotherapy benefits subgroups of breast cancer patients with T1-2 tumor and 1-3 axillary lymph node(s) metastasis. Radiol Oncol. 2014; 48:314–322. PMID: 25177247.

23. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008; 26:3324–3330. PMID: 18612148.

24. Cheng JC, Chen CM, Liu MC, Tsou MH, Yang PS, Jian JJ, et al. Locoregional failure of postmastectomy patients with 1-3 positive axillary lymph nodes without adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2002; 52:980–988. PMID: 11958892.

25. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009; 27:1062–1068. PMID: 19164210.

26. Gaffney DK, Tsodikov A, Wiggins CL. Diminished survival in patients with inner versus outer quadrant breast cancers. J Clin Oncol. 2003; 21:467–472. PMID: 12560437.

27. Shen SC, Liao CH, Lo YF, Tsai HP, Kuo WL, Yu CC, et al. Favorable outcome of secondary axillary dissection in breast cancer patients with axillary nodal relapse. Ann Surg Oncol. 2012; 19:1122–1128. PMID: 21969085.

28. Biancosino A, Bremer M, Karstens JH, Biancosino C, Meyer A. Postoperative periclavicular radiotherapy in breast cancer patients with 1-3 positive axillary lymph nodes: outcome and morbidity. Strahlenther Onkol. 2012; 188:417–423. PMID: 22410836.
29. Viani GA, Godoi da Silva LB, Viana BS. Patients with N1 breast cancer: who could benefit from supraclavicular fossa radiotherapy? Breast. 2014; 23:749–753. PMID: 25231194.

30. Olivotto IA, Truong PT, Chua B. Postmastectomy radiation therapy: who needs it? J Clin Oncol. 2004; 22:4237–4239. PMID: 15452185.

Figure 1
Kaplan-Meier estimates (log-rank test) for locoregional recurrence (A), locoregional recurrence-free survival (B), and breast cancer-specific survival (C), according to molecular subtypes.
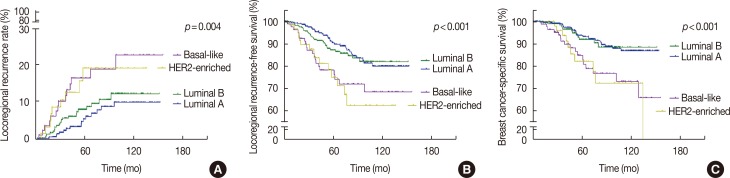
Figure 2
Kaplan-Meier estimates (log-rank test) for locoregional recurrence (A), locoregional recurrence-free survival (B), and breast cancer-specific survival (C), according to risk groups.
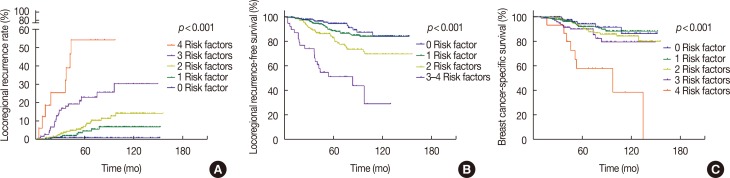
Table 1
Univariate analysis for clinicopathologic characteristics
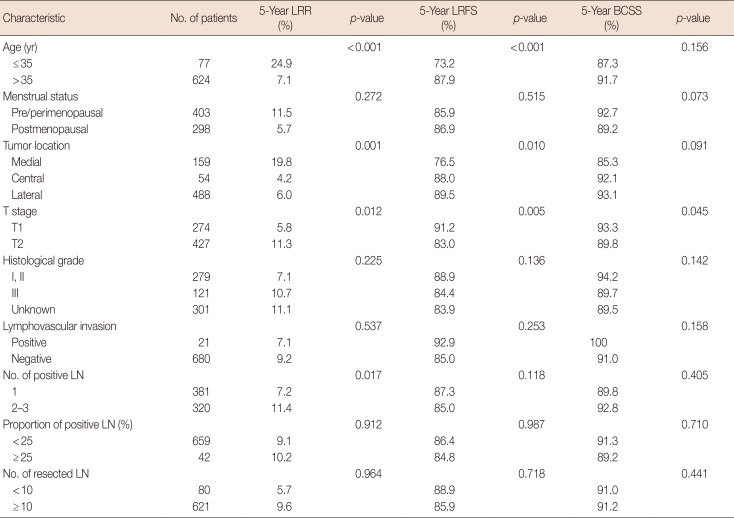
Table 2
Distribution of clinicopathologic characteristics among molecular subtypes
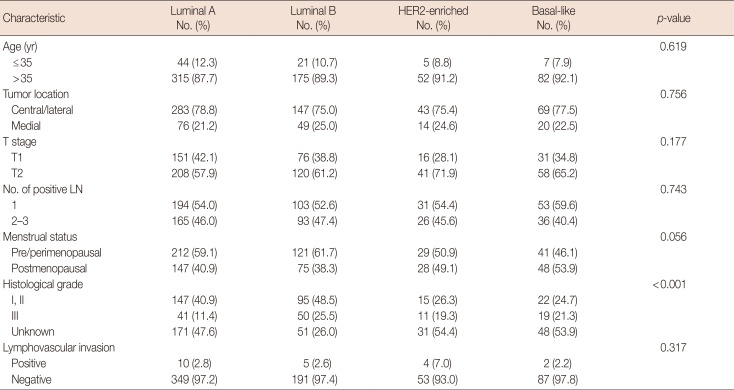
Table 3
Univariate analysis for molecular subtypes
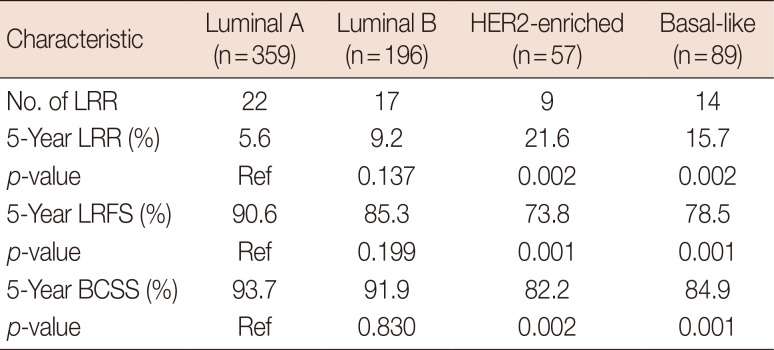
Table 4
Multivariate analysis of prognostic factors




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download