Abstract
The mutations in the dual oxidase 2 (DUOX2) and dual oxidase maturation factor 2 (DUOXA2) genes can cause congenital hypothyroidism (CH). This study reports the pedigree with goitrous congenital hypothyroidism (GCH) due to the coexistence of heterozygous mutations in the DUOX2 and DUOXA2 genes. The two sisters with GCH were diagnosed with CH at neonatal screening and were enrolled in this study. The DUOX2, DUOXA2, and thyroid peroxidase (TPO) genes were considered for genetic defects screening. Family members of the patients and normal controls were also enrolled and evaluated. The two girls harbored compound heterozygous mutations, including a new mutation of c.2654G>T (p.R885L) in the maternal DUOX2 allele and c.738C>G (p.Y246X) in the paternal DUOXA2 allele, that has been previously reported. The germline mutations from the families were consistent with an autosomal recessive inheritance pattern. No mutations in the TPO gene and the controls were observed.
The genetic pathogenesis remains unclear in most congenital hypothyroidism (CH) cases. Familial cases are associated with genetic defects to a certain extent, approximately 20% CH cases heing caused by inherited defects in the pathway of thyroid hormone synthesis.12
A critical step in the synthesis of thyroid hormone is the generation of hydrogen peroxide (H2O2). H2O2 is produced by dual oxidase (DUOX)/dual oxidase maturation factor (DUOXA) at the apical membrane of follicular thyroid cells, and is used as a substrate by thyroid peroxidase (TPO) in the organification of iodide and incorporation of iodine into thyroglobulin. DUOXA is essential for DUOX maturation and activation, thereby determining normal DUOX function.34 To date, mutations in the DUOX2/DUOXA2 system have been identified to be associated with CH, and the system is one of the important candidate genes for CH.56789 In addition, The most prevailing causes of goitrous congenital hypothyroidism (GCH) are TPO defects. Until now, more than 90 inactivating mutations in the TPO gene have been reported.2101112 These mutations establish a heterogeneous spectrum of GCH, with an autosomal recessive mode of inheritance.
Currently, the genetic defects in CH are not fully understood. In this study, the DUOX2, DUOXA2, and TPO genes were considered for screening genetic defects in a girl and her younger sister with GCH.
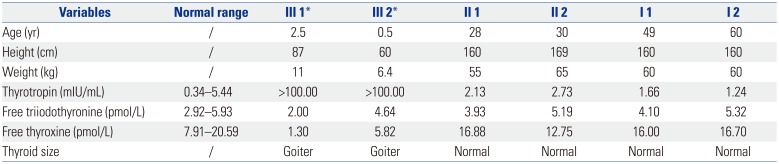
The girl and her sister with GCH were recruited for molecular analysis of mutations. CH was diagnosed on the basis of serum levels of thyrotropin, free thyroxine and free triiodothyronine at the neonatal screening stage. Thyroid ultrasound demonstrated that both the girl and her sister had goiter. Their parents were not consanguineous and had normal thyroid function (Table 1). The girls were regularly followed up. Until now, they had no obvious complications. This study was approved by the Ethics Committee of the hospital. Written informed consent was obtained.
Venous blood samples were obtained from the girls. Genomic DNA was extracted by using the phenol-chloroform extraction method from peripheral blood leukocytes. Primers were designed to target the flanking intron regions of the exons. All exons of the DUOX2 (MIM# 606759, GenBank NM_014080.4), DUOXA2 (MIM# 612772, GenBank NM_207581.3), and TPO (MIM# 606765, GenBank NM_000547.5) genes were amplified by PCR. PCR products were purified and Sanger sequenced directly for variance analysis. If an mutation was identified, the target fragment was also amplified in the girl's parents and in 105 control individuals.
As shown in Fig. 1, the genetic analysis demonstrated that the two girls carried the same compound heterozygous mutations: G to T transversion at 2654 position [c.2654G>T (p. Arg885Leu, p.R885L)] in the exon 20 of the DUOX2 gene and C to G transversion at nucleotide position 738 [c.738C>G (p.Y246X)] in the exon 5 of DUOXA2 gene.
The missense mutation p.R885L is presented in 1000 Genomes Project database, but not found in Pubmed and controls. Bioinformatics analysis with Mutation Taster, PolyPhen-2 and PROVEAN revealed that the p.R885L mutation is capable of causing disease (Mutation Taster score of 120, prob ≥0.99), is most likely damaging (PolyPhen-2 score of 0.993), or is likely to have deleterious effects (PROVEAN score of -6.040).
Heterozygous mutations were detected in the family members. Pedigree analysis showed that the pattern was consistent with autosomal recessive inheritance. No mutations in the TPO gene was detected in our pedigree. None of the controls showed the same pathogenic variants.
In the present study, mutations in the DUOX2, DUOXA2, and TPO genes were screened in the two patients with GCH. The compound heterozygous mutations, p.R885L in the DUOX2 gene and p.Y246X in the DUOXA2, were detected. Pedigree analysis revealed an autosomal recessive inheritance pattern.
Mutations in the DUOX2/DUOXA2 heterodimer are the common genetic defects of CH. Previous studies reported four mutations in the DUOXA2 (p.I26M, p.Y138X, p.C189R, and p.Y246X) associated with CH. The patients with homozygous nonsense mutation (p.Y246X) exhibited mild permanent CH and goiter, which is similar to our patients. The p.Y246X mutation of DUOXA2 leads to a complete loss of function in the ho-mozygous patient, thus ensuring to a secondary deficiency of DUOX2 activity.13 Together with our results, the findings indicate that the p.Y246X mutation frequently occurs in Chinese population,1314 and the frequency of affected homozygous for p.Y246X is estimated to be one of 34000 newborns in chinese population.15
To date, over 40 mutations in the DUOX2 gene have been described to be correlated with CH.13 Maruo, et al.8 firstly reported the p.R885Q (c.2654G>A) mutation in the DUOX2 gene. However, the patients in this study was found to have G to T transversion (c.2654G>T) at the same position, resulting in the replacement of an acidic residue at amino acid position 885 (p.Arg885Leu, p.R885L). In silico analysis revealed that this alteration (p.R885L) affected the structure/function of the DUOX2 protein and were capable of causing disease. To our best knowledge, p.R885L is the first ever reported in patients with CH. In addition, the two girls in our study presented with a normally located but enlarged thyroid gland. Their parents, each with a single heterozygous mutation, exhibited normal thyroid function. Our patients in the present study developed no physical or cognitive developmental defects; Timely and effective treatment contributed to the satisfactory results.
In conclusion, the present study identified two missense mutations in two patients with CH: c.2654G>T (p.R885L) in the DUOX2 gene and c.738C>G (p.Y246X) in the DUOXA2. The findings indicate the importance of molecular diagnosis and classification of CH.
ACKNOWLEDGEMENTS
This work was supported by the Social Development Project of Huai'an City (grant number: HAS2014005 and HAS201610) and the Science and Technology Research Projects of Guangxi University (grant number: KY2015LX267).
References
1. Anjum A, Afzal MF, Iqbal SM, Sultan MA, Hanif A. Congenital hypothyroidism in neonates. Indian J Endocrinol Metab. 2014; 18:213–216. PMID: 24741519.
2. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014; 28:133–150. PMID: 24629857.

3. Corvilain B, van Sande J, Laurent E, Dumont JE. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology. 1991; 128:779–785. PMID: 1846588.

4. Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006; 281:18269–18272. PMID: 16651268.
5. Grasberger H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol. 2010; 322:99–106. PMID: 20122987.

6. Yi RH, Zhu WB, Yang LY, Lan L, Chen Y, Zhou JF, et al. A novel dual oxidase maturation factor 2 gene mutation for congenital hypothyroidism. Int J Mol Med. 2013; 31:467–470. PMID: 23292166.

7. Wang F, Lu K, Yang Z, Zhang S, Lu W, Zhang L, et al. Genotypes and phenotypes of congenital goitre and hypothyroidism caused by mutations in dual oxidase 2 genes. Clin Endocrinol (Oxf). 2014; 81:452–457. PMID: 24735383.

8. Maruo Y, Takahashi H, Soeda I, Nishikura N, Matsui K, Ota Y, et al. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J Clin Endocrinol Metab. 2008; 93:4261–4267. PMID: 18765513.

9. Hulur I, Hermanns P, Nestoris C, Heger S, Refetoff S, Pohlenz J, et al. A single copy of the recently identified dual oxidase maturation factor (DUOXA) 1 gene produces only mild transient hypothyroidism in a patient with a novel biallelic DUOXA2 mutation and monoallelic DUOXA1 deletion. J Clin Endocrinol Metab. 2011; 96:E841–E845. PMID: 21367925.

10. Niu DM, Hwang B, Chu YK, Liao CJ, Wang PL, Lin CY. High prevalence of a novel mutation (2268 insT) of the thyroid peroxidase gene in Taiwanese patients with total iodide organification defect, and evidence for a founder effect. J Clin Endocrinol Metab. 2002; 87:4208–4212. PMID: 12213873.

11. Ris-Stalpers C, Bikker H. Genetics and phenomics of hypothyroidism and goiter due to TPO mutations. Mol Cell Endocrinol. 2010; 322:38–43. PMID: 20153806.

12. Ma SG, Wu XJ, Liu H, Xu W, He L. Mutations of the thyroid peroxidase gene in Chinese siblings with congenital goitrous hypothyroidism. Arq Bras Endocrinol Metabol. 2012; 56:614–617. PMID: 23329183.

13. O'Neill S, Brault J, Stasia MJ, Knaus UG. Genetic disorders coupled to ROS deficiency. Redox Biol. 2015; 6:135–156. PMID: 26210446.
14. Wang F, Lu K, Yang Z, Zhang S, Lu W, Zhang L, et al. Genotypes and phenotypes of congenital goitre and hypothyroidism caused by mutations in dual oxidase 2 genes. Clin Endocrinol (Oxf). 2014; 81:452–457. PMID: 24735383.

15. Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008; 93:605–610. PMID: 18042646.

Fig. 1
Genotypes indicating the pedigrees with congenital hypothyroidism due to the coexistence of heterozygous mutations, c.2654G>T (p.R885L) in the DUOX2 gene and c.738C>G (p.Y246X) in the DUOXA2.

Table 1
Thyroid Blood Tests and Clinical Data of the Family in September 2015




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download