Abstract
Objectives
To investigate the effects of microelectric treatment by transcutaneous electrical nerve stimulation (TENS) on functional recovery and histological changes in a rat model of spinal cord injury (SCI).
Summary of Literature Review
The effects of TENS on spasticity and its underlying mechanisms remain unclear.
Materials and Methods
SCI was induced by a 1.5-mm impactor with 200,000–260,000 dyne after laminectomy. Rats were divided into the following groups: group I (normal control), group II (microelectric treatment of 0 A), group III (microelectric treatment of 100 μA for 1 hr/day), group IV (microelectric treatment of 400 μA for 1 hr/day), and group V (microelectric treatment of 400 μA for 24 hr/day). After inducing SCI, rats were assessed by a sensory test with von Frey filaments and the locomotor recovery test (BBB rating scale) at 1, 4, 7, 14, 21, and 28 days. To evaluate spinal cord damage, histopathological studies were performed with hematoxylin and eosin. Brain-derived neurotrophic factor (BDNF) and TrkB immunohistochemistry studies were performed at 28 days.
Results
In groups IV and V, the BBB score had significantly improved on days 21 and 28 after SCI, and the TENS-treated groups showed significant neuronal recovery. After SCI, groups IV and V showed a significant recovery of locomotor function and the motor sensory response of the withdrawal threshold to 3.5 g. In addition, necrotic tissue and cystic spaces in the spinal cord were significantly reduced and BDNF/TrkB-positive cells were highly expressed in groups III, IV, and V.
Go to : 
REFERENCES
1. Zhang J, Feng G, Bao G, et al. Nuclear translocation of PKM2 modulates astrocyte proliferation via p27 and -catenin pathway after spinal cord injury. Cell Cycle. 2015; 14(16):2609–18. DOI: 10.1080/15384101. 2015.1064203.

2. The National SCI Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2013 Jan; 36(1):1–2. DOI: 10.1179/1079026813Z.000000000136.
3. Yip PK, Malaspina A. Spinal cord trauma and the molecu-lar point of no return. Mol Neurodegener. 2012 Feb 8; 7:6. DOI: 10.1186/1750-1326-7-6.

4. Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004 Jul-Aug; 4(4):451–64. DOI: 10.1016/j.spinee.2003.07.007.

5. Liu XZ, Xu XM, Hu R, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997 Jul 15; 17(14):5395–406.

6. Huan W, Wu X, Zhang S, et al. Spatiotemporal patterns and essential role of TNF receptor-associated factor 5 expression after rat spinal cord Injury. J Mol Histol. 2012 Oct; 43(5):527–33. DOI: 10.1007/s10735-012-9411-5.

7. Crowe MJ, Bresnahan JC, Shuman SL, et al. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997 Jan; 3(1):73–6. DOI: DOI:10.1038/nm0197-73.

8. Liu J, Wu W, Hao J, et al. PRDM5 Expression and Essential Role After Acute Spinal Cord Injury in Adult Rat. Neu-rochem Res. 2016 Dec; 41(12):3333–43. DOI: 10.1007/s11064-016-2066-y.

9. Zhang J, Li D, Shen A, et al. Expression of RBMX after spinal cord injury in rats. J Mol Neurosci. 2013 Feb; 49(2):417–29. DOI: 10.1007/s12031-012-9914-2.

10. Katoh K, Ikata T, Katoh S, et al. Induction and its spread of apoptosis in rat spinal cord after mechanical trauma. Neurosci Lett. 1996 Sep 20; 216(1):9–12. DOI: DOI:10.1016/s0304-3940 (96)12999-2.

11. Cruccu G, Aziz TZ, Garcia-Larrea L, et al. EFNS guide-lines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007 Sep; 14(9):952–70. DOI: 10.1111/j.1468-1331.2007.01916.x.

12. Carroll D, Moore RA, McQuay HJ, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2001; 3:CD003222. DOI: 10.1002/14651858.CD003222.

13. Baptista AF, Gomes JR, Oliveira JT, et al. High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J Peripher Nerv Syst. 2008 Mar; 13(1):71–80. DOI: 10.1111/j.1529-8027.2008.00160.x.

14. Tashani O, Johnson M. Transcutaneous Electrical Nerve Stimulation (TENS) A Possible Aid for Pain Relief in De-veloping Countries? Libyan J Med. 2009 Jun 1; 4(2):62–5. DOI: 10.4176/090119.

15. Krishna V, Andrews H, Jin X, et al. A contusion model of severe spinal cord injury in rats. J Vis Exp. 2013 Aug 17; 78:). DOI: 10.3791/50111.

16. You JW, Sohn HM, Park SH. Diminution of second-ary injury after administration of pharmacologic agents in acute spinal cord injury rat model -comparison of statins, erythropoietin and polyethylene glycol-. J Korean Soc Spine Surg. 2012; 19(3):77–84. DOI: DOI:10.4184/jkss.2012.19.3.77.

17. Inoue T, Takenoshita M, Shibata M, et al. Long-lasting effect of transcutaneous electrical nerve stimulation on the thermal hyperalgesia in the rat model of peripheral neuropathy. J Neurol Sci. 2003 Jul 15; 211(1-2):43–7. DOI: DOI:10.1016/s0022-510x(03)00038-8.

18. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reli-able locomotor rating scale for open field testing in rats. J Neurotrauma. 1995 Feb; 12(1):1–21. DOI: 10.1089/neu.1995.12.1.

19. Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative as-sessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994 Jul; 53(1):55–63. DOI: DOI:10.1016/0165-0270 (94)90144-9.

20. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–62. DOI: 10.1146/annurev.pa.20.040180.002301.

21. Martin R, Sadowsky C, Obst K, et al. Functional electrical stimulation in spinal cord injury:: from theory to practice. Top Spinal Cord Inj Rehabil. 2012 Winter; 18(1):28–33. DOI: 10.1310/sci1801-28.

22. Park J, Seo D, Choi W, et al. The effects of exercise with TENS on spasticity, balance, and gait in patients with chronic stroke: a randomized controlled trial. Med Sci Monit. 2014 Oct 10; 20:1890–6. DOI: 10.12659/MSM.890926.

23. Solak O, Emmiler M, Ela Y, et al. Comparison of continuous and intermittent transcutaneous electrical nerve stimulation in postoperative pain management after coronary artery bypass grafting: a randomized, placebo-controlled prospective study. Heart Surg Forum. 2009 Oct; 12(5):E266–71. DOI: 10.1532/HSF98.20081139.

24. Cohen-Cory S, Kidane AH, Shirkey NJ, et al. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010 Apr; 70(5):271–88. DOI: 10.1002/dneu.20774.

25. Hahm SC, Yoon YW, Kim J. High-frequency transcutaneous electrical nerve stimulation alleviates spasticity after spinal contusion by inhibiting activated microglia in rats. Neurorehabil Neural Repair. 2015 May; 29(4):370–81. DOI: 10.1177/1545968314545172.

26. McCall J, Weidner N, Blesch A. Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res. 2012 Jul; 349(1):27–37. DOI: 10.1007/s00441-012-1388-6.

27. Allen SJ, Dawbarn D, Eckford SD, et al. Cloning of a non-catalytic form of human trkB and distribution of mes-senger RNA for trkB in human brain. Neuroscience. 1994 Jun; 60(3):825–34. DOI: DOI:10.1016/0306-4522 (94)90507-x.
28. Sandhya VK, Raju R, Verma R, et al. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal. 2013 Dec; 7(4):301–7. DOI: 10.1007/s12079-013-0200-z.

29. Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004 Sep; 22(3):123–31. DOI: 10.1080/08977190410001723308.

Go to : 
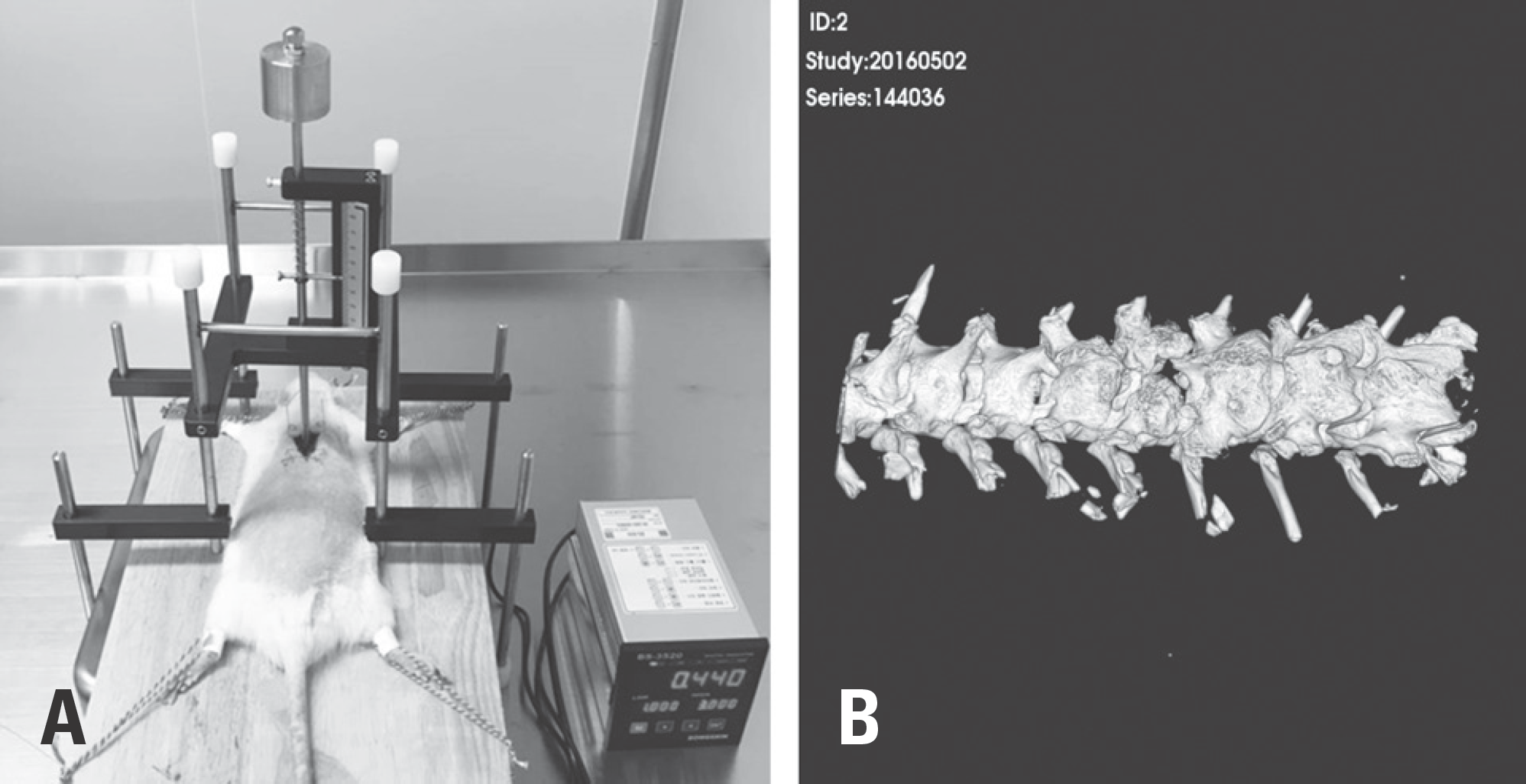 | Fig. 1.Establishment of the spinal cord injury (SCI) model in the rats and confirmation of T9-10 laminectomy. (A) Establishment of the SCI model in the rats using the Modified Ohio Impactor. (B) Confirmation of T9-10 lesions using micro-computed tomography. |
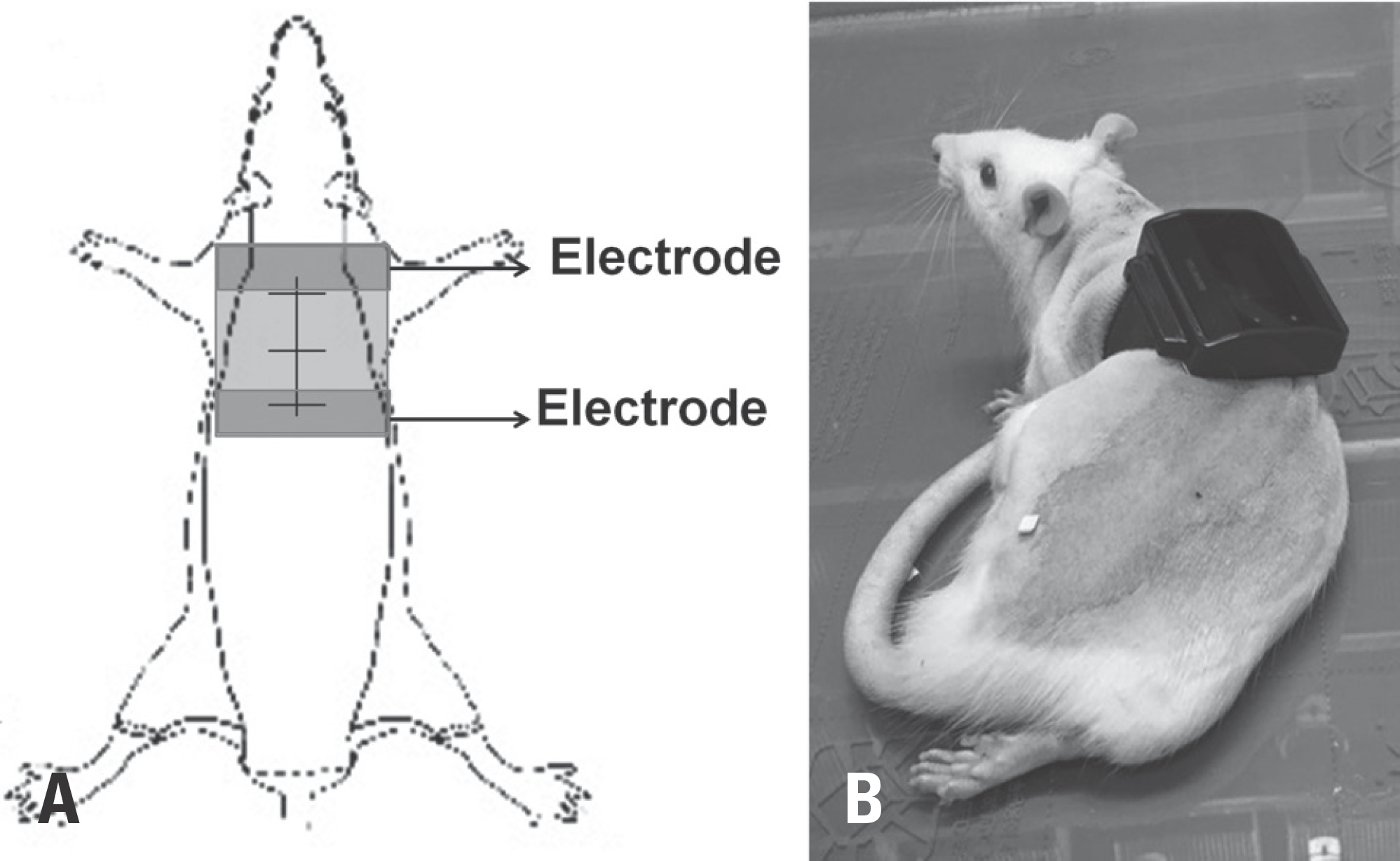 | Fig. 2.Transcutaneous electrical nerve stimulation (TENS) treatment in the rat model of spinal cord injury (SCI). (A) Schematic representation of the rat model system for TENS treatment through 2 coated electrodes located below the instrument. (B) Constant current stimuli were applied to the dorsal surface of the SCI rats. |
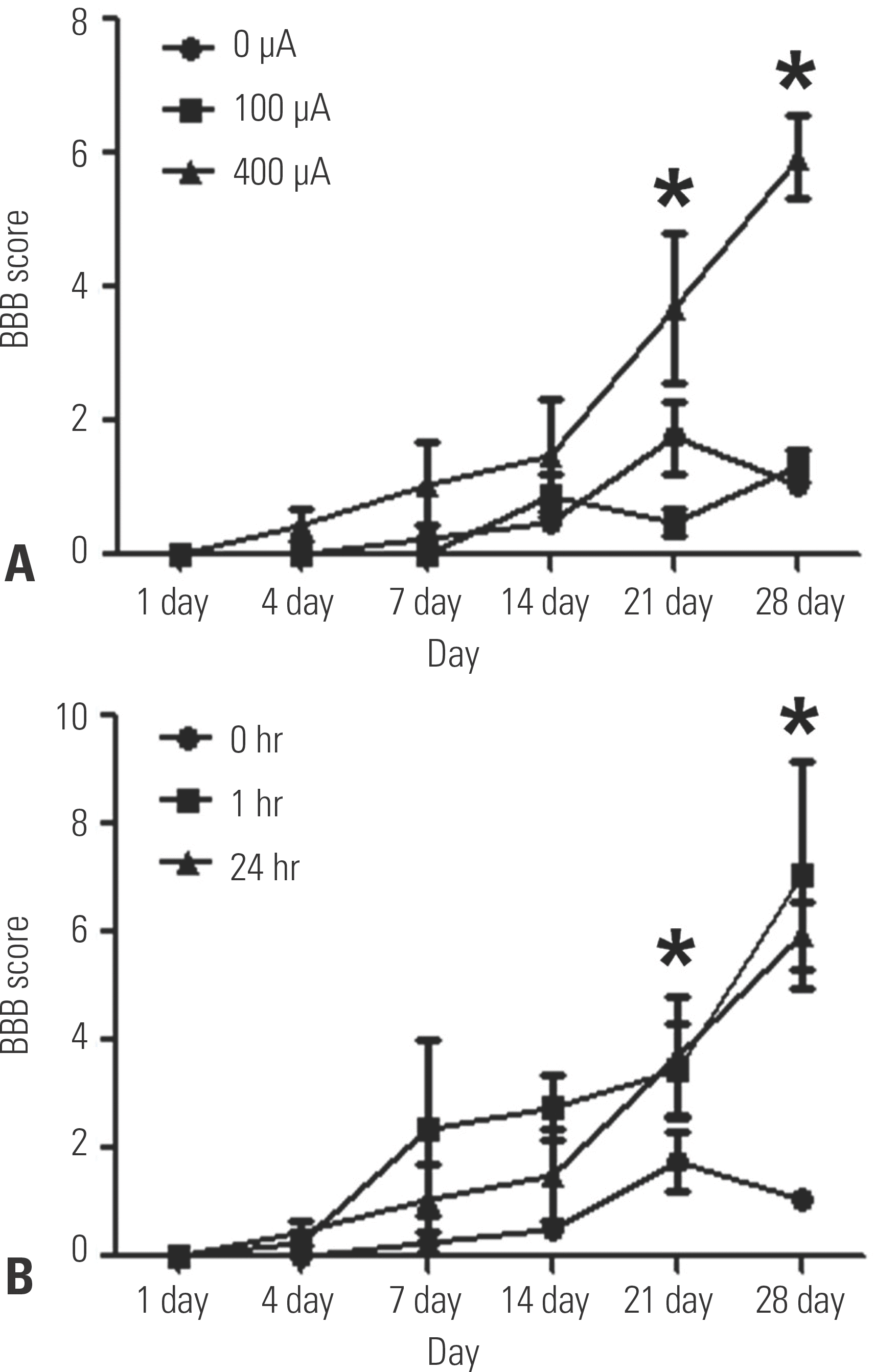 | Fig. 3.Locomotor recovery test using BBB scoring. (A) Transcutaneous electrical nerve stimulation (TENS)-treated animals (400 μA) showed better BBB locomotor scores than the animals treated with 0 A (∗p<0.05). (B) TENS treatment for 1 hr/day and 24 hr/day improved BBB locomotor scores more than 0 hr/day of treatment (∗p<0.05). |
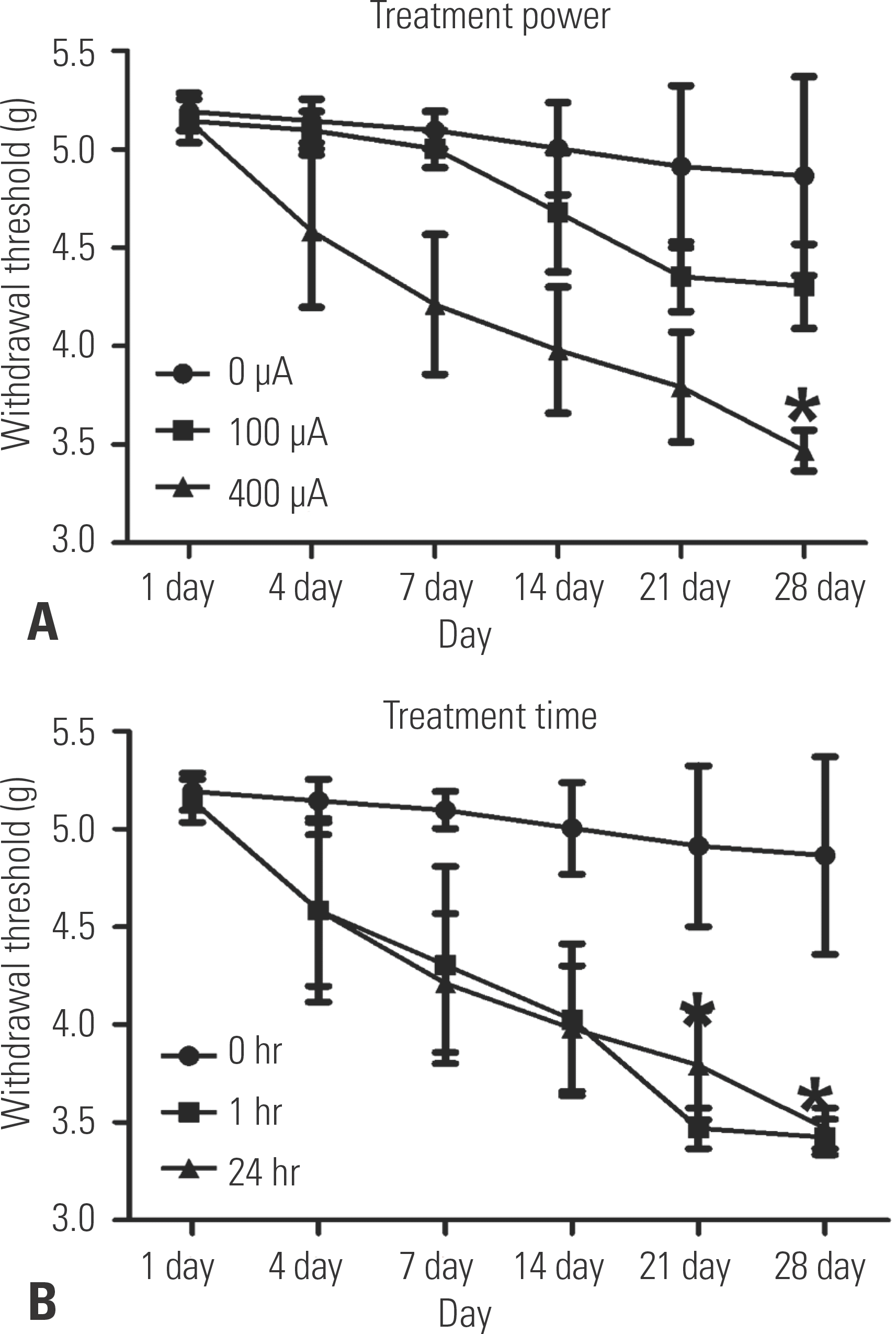 | Fig. 4.Motor sensory response test by the 50% withdrawal threshold through von Frey filament application. (A) Transcutaneous electrical nerve stimulation (TENS)-treated animals (400 μA) had a lower 50% withdrawal threshold than the animals treated with 0 A (∗p<0.05). (B) TENS treatment for 1 hr/day and 24 hr/day led to a lower 50% withdrawal threshold than 0 hr/day of treatment (∗p<0.05). |
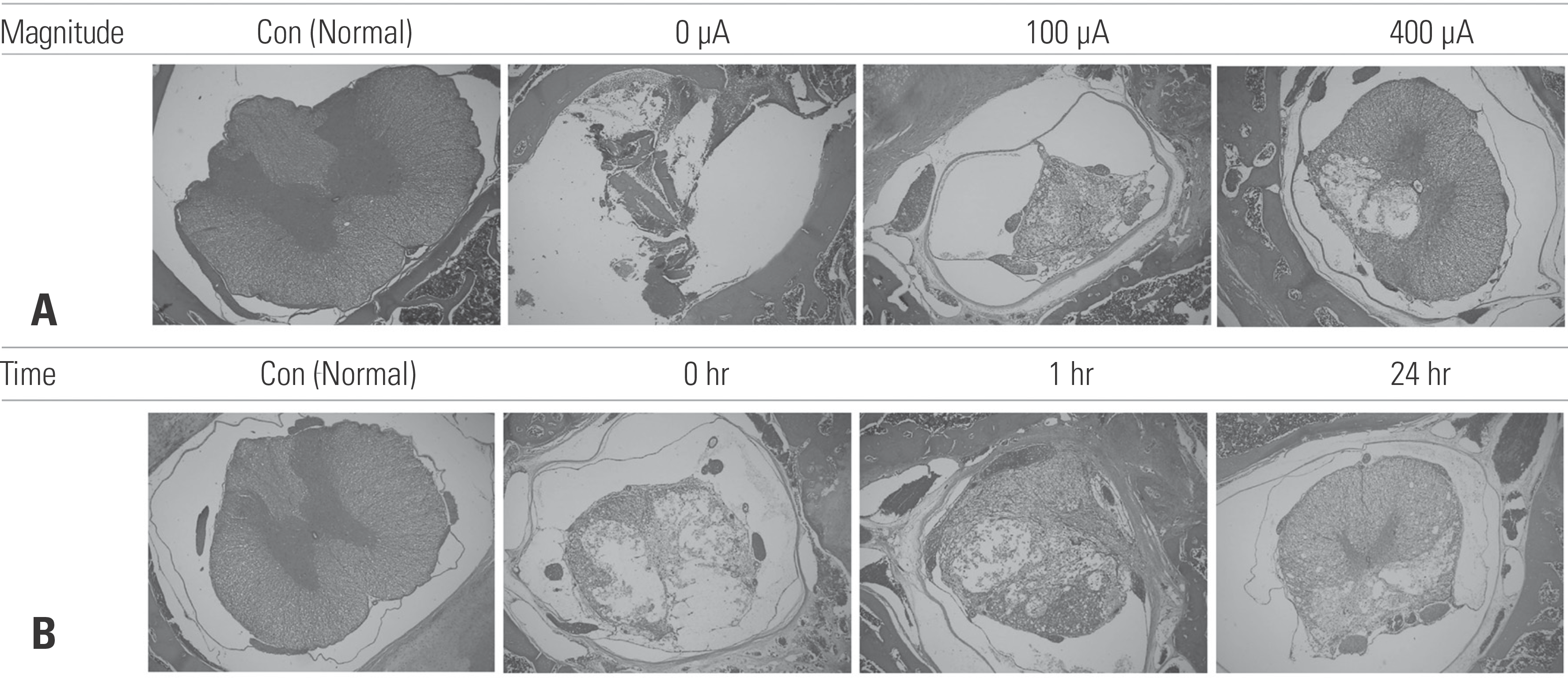 | Fig. 5.Photographs taken from the site of a spinal cord injury, excised 28 days after the injury. The spinal cord segments were cut into 8-μm axial sections. Lesion swelling is seen on low-power magnification (×40) with hematoxylin and eosin staining. (A) Transcutaneous electrical nerve stimulation (TENS)-treated animals (400 μA) showed better spinal cord recovery than the animals treated with 0 A. (B) TENS treatment for 1 hr/day and 24 hrs/day favored spinal cord recovery in comparison with 0 hr/day of treatment. |
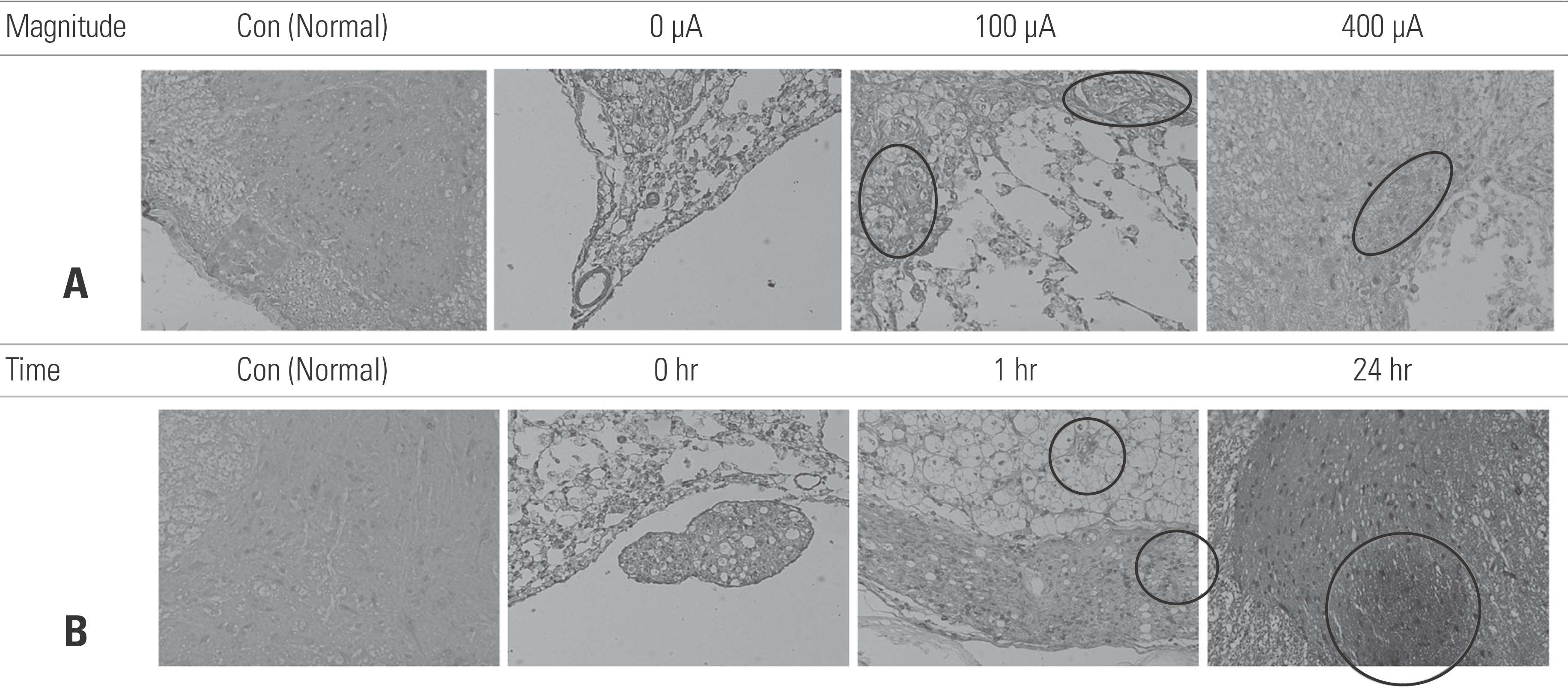 | Fig. 6.Immunohistochemical study of brain-derived neurotrophic factor (BDNF) expression at the site of a spinal cord injury, excised 28 days after the injury. Each field was examined at ×100 (10 objective × 10 ocular) magnification. (A) Transcutaneous electrical nerve stimulation (TENS)-treated animals (400 μA) showed higher BDNF expression than the animals treated with 0 A. (B) TENS treatment for 1 hr/day and 24 hr/day led to higher BDNF expression than 0 hr/day of treatment. |
 | Fig. 7.Immunohistochemical study of TrkB expression at the site of a spinal cord injury, excised 28 days after the injury. Each field was examined at ×100 (10 objective × 10 ocular) magnification. (A) Transcutaneous electrical nerve stimulation (TENS)-treated animals (400 μA) showed higher TrkB expression than the animals treated with 0 A. (B) TENS treatment for 1 hr/day and 24 hr/day led to higher TrkB expression than 0 hr/day of treatment. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download