1. Cheitlin MD. Cardiovascular physiology: changes with aging. Am J Geriatr Cardiol. 2003; 12:9–13. PMID:
12502909.
2. Smeltzer SC, Bare B, Hinkle JL, Cheever KH. Brunner and Suddarth's textbook of medical-surgical nursing. Philadelphia, PA: Lippincott Williams & Wilkins;2010.
3. Paneni F, Diaz Canestro C, Libby P, Luscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017; 69:1952–1967. PMID:
28408026.
4. Rakesh B, Suresha B, Varghese AR, Joy ET. Analysis of prevalence and risk factors for cardiovascular disorders. J Pharm Pharm Sci. 2016; 5:1361–1373.
5. Pham T, Sulejmani F, Shin E, Wang D, Sun W. Quantification and comparison of the mechanical properties of four human cardiac valves. Acta Biomater. 2017; 54:345–355. PMID:
28336153.
6. Schoen FJ. Morphology, clinicopathologic correlations, and mechanisms in heart valve health and disease. Cardiovasc Eng Technol. 2018; 9:126–140. PMID:
27502286.
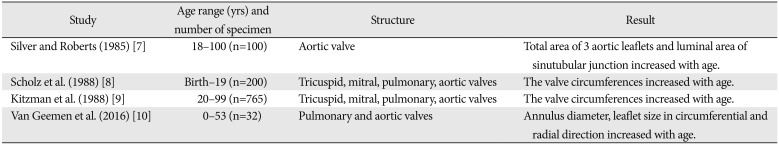
7. Silver MA, Roberts WC. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. Am J Cardiol. 1985; 55:454–461. PMID:
3155899.
8. Scholz DG, Kitzman DW, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part I (Growth): a quantitative anatomic study of 200 specimens from subjects from birth to 19 years old. Mayo Clin Proc. 1988; 63:126–136. PMID:
3276973.
9. Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988; 63:137–146. PMID:
3276974.
10. van Geemen D, Soares AL, Oomen PJ, Driessen-Mol A, Janssenvan den Broek MW, van den Bogaerdt AJ, Bogers AJ, Goumans MJ, Baaijens FP, Bouten CV. Age-dependent changes in geometry, tissue composition and mechanical properties of fetal to adult cryopreserved human heart valves. PLoS One. 2016; 11:e0149020. PMID:
26867221.
11. Sell S, Scully RE. Aging changes in the aortic and mitral valves: histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol. 1965; 46:345–365. PMID:
14270114.
12. McDonald PC, Wilson JE, McNeill S, Gao M, Spinelli JJ, Rosenberg F, Wiebe H, McManus BM. The challenge of defining normality for human mitral and aortic valves: geometrical and compositional analysis. Cardiovasc Pathol. 2002; 11:193–209. PMID:
12140125.
13. Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006; 113:1344–1352. PMID:
16534030.
14. Ormiston JA, Shah PM, Tei C, Wong M. Size and motion of the mitral valve annulus in man. I. A two-dimensional echocardiographic method and findings in normal subjects. Circulation. 1981; 64:113–120. PMID:
7237707.
15. Krishnaiah M, Mrudula C. Morphometric study of mitral valve orifice an echo cardiographic study. Int J Pharma Bio Sci. 2011; 2:181–187.
16. Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011; 73:29–46. PMID:
20809794.
17. Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006; 34:1799–1819. PMID:
17053986.
18. Misfeld M, Sievers HH. Heart valve macro- and microstructure. Philos Trans R Soc Lond B Biol Sci. 2007; 362:1421–1436. PMID:
17581807.
19. Anderson RH, Ho SY, Becker AE. Anatomy of the human atrioventricular junctions revisited. Anat Rec. 2000; 260:81–91. PMID:
10967539.
20. Anderson RH. Clinical anatomy of the aortic root. Heart. 2000; 84:670–673. PMID:
11083753.
21. Athavale S, Deopujari R, Sinha U, Lalwani R, Kotgirwar S. Is tricuspid valve really tricuspid? Anat Cell Biol. 2017; 50:1–6. PMID:
28417048.
22. Zimmerman J, Bailey CP. Surgical significance of fibrous skeleton of heart. J Thorac Cardiovasc Surg. 1962; 44:701.
23. Xanthos T, Dalivigkas I, Ekmektzoglou KA. Anatomic variations of the cardiac valves and papillary muscles of the right heart. Ital J Anat Embryol. 2011; 116:111–126. PMID:
22303639.
24. Silver MD, Lam JH, Ranganathan N, Wigle ED. Morphology of the human tricuspid valve. Circulation. 1971; 43:333–348. PMID:
5544987.
25. Khan AA, Ali II, Karim MA. Anatomy of the human tricuspid valve. Pak Heart J. 2012; 19:39–43.
26. Aktas EO, Govsa F, Kocak A, Boydak B, Yavuz IC. Variations in the papillary muscles of normal tricuspid valve and their clinical relevance in medicolegal autopsies. Saudi Med J. 2004; 25:1176–1185. PMID:
15448762.
27. Joudinaud TM, Flecher EM, Duran CM. Functional terminology for the tricuspid valve. J Heart Valve Dis. 2006; 15:382–388. PMID:
16784076.
28. Duplessis LA, Marchand P. The anatomy of the mitral valve and its associated structures. Thorax. 1964; 19:221–227. PMID:
14143500.
29. Ranganathan N, Lam JH, Wigle ED, Silver MD. Morphology of the human mitral valve. II. The value leaflets. Circulation. 1970; 41:459–467. PMID:
5415983.
30. McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr. 2010; 11:i3–i9. PMID:
21078837.
31. Salgo IS, Gorman JH 3rd, Gorman RC, Jackson BM, Bowen FW, Plappert T, St John Sutton MG, Edmunds LH Jr. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002; 106:711–717. PMID:
12163432.
32. Gross L, Kugel MA. Topographic anatomy and histology of the valves in the human heart. Am J Pathol. 1931; 7:445–474. PMID:
19969978.
33. Bateman MG, Quill JL, Hill AJ, Iaizzo PA. The anatomy and function of the semilunar valves. In : Iaizzo PA, Bianco RW, Hill AJ, St. Louis JD, editors. Heart Valves: From Design to Clinical Implantation. New York: Springer;2013. p. 27–43.
34. Muriago M, Sheppard MN, Ho SY, Anderson RH. Location of the coronary arterial orifices in the normal heart. Clin Anat. 1997; 10:297–302. PMID:
9283725.
35. Henry WL, Ware J, Gardin JM, Hepner SI, McKay J, Weiner M. Echocardiographic measurements in normal subjects. Growth-related changes that occur between infancy and early adulthood. Circulation. 1978; 57:278–285. PMID:
618615.
36. Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980; 62:1054–1061. PMID:
7418156.
37. Roge CL, Silverman NH, Hart PA, Ray RM. Cardiac structure growth pattern determined by echocardiography. Circulation. 1978; 57:285–290. PMID:
618616.
38. Prakash R, Umali SA. Comparison of echocardiographic and necropsy measurements of left ventricular wall thickness in patients with coronary artery disease. Am J Cardiol. 1984; 53:838–841. PMID:
6702634.
39. Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee. (Stroke Statistics Subcommittee):Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016; 133:e38–e360. PMID:
26673558.
40. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28–e292. PMID:
24352519.
41. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017; 135:e146–e603. PMID:
28122885.
42. Buja LM, Butany J. Cardiovascular pathology. 4th ed. London:: Elsevier Science;2015.
43. Butcher JT, Mahler GJ, Hockaday LA. Aortic valve disease and treatment: the need for naturally engineered solutions. Adv Drug Deliv Rev. 2011; 63:242–268. PMID:
21281685.
44. Virmani R, Atkinson JB, Fenoglio JJ. Cardiovascular pathology. Philadelphia, PA: WB Saunders Company;1991.
45. Leask RL, Jain N, Butany J. Endothelium and valvular diseases of the heart. Microsc Res Tech. 2003; 60:129–137. PMID:
12539167.
46. Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011; 8:162–172. PMID:
21263455.
47. Webb J, Arden C, Chambers JB. Heart valve disease in general practice: a clinical overview. Br J Gen Pract. 2015; 65:e204–e206. PMID:
25733443.
48. Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease: current management and future challenges. Lancet. 2016; 387:1324–1334. PMID:
27025438.
49. Grunkemeier GL, Li HH, Starr A. Heart valve replacement: a statistical review of 35 years' results. J Heart Valve Dis. 1999; 8:466–470. PMID:
10517384.
50. Lupinetti FM, Warner J, Jones TK, Herndon SP. Comparison of human tissues and mechanical prostheses for aortic valve replacement in children. Circulation. 1997; 96:321–325. PMID:
9236452.
51. Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2003; 41:893–904. PMID:
12651032.
52. Jamieson WR, von Lipinski O, Miyagishima RT, Burr LH, Janusz MT, Ling H, Fradet GJ, Chan F, Germann E. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after mitral valve replacement. J Thorac Cardiovasc Surg. 2005; 129:1301–1308. PMID:
15942570.
53. Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000; 36:1152–1158. PMID:
11028464.
54. Hasan A, Ragaert K, Swieszkowski W, Selimović S, Paul A, Camci-Unal G, Mofrad MR, Khademhosseini A. Biomechanical properties of native and tissue engineered heart valve constructs. J Biomech. 2014; 47:1949–1963. PMID:
24290137.
55. Gupta C, Shetti VR, Manju BV. Dimensions of the human adult mitral valve in the embalmed cadaver. J Morphol Sci. 2013; 30:6–10.
56. Roy S, Saha A. Mitral valve beyond classical view: a morphometric evaluation. J Anat Soc India. 2016; 65:20–23.
57. Udhayakumar S, Yasawardene S. Morphological and morphometric analysis of mitral valve leaflets in a Sri Lankan population fresh autopsy study. In : Annual International Conference on Microscopic and Macroscopic Anatomy; 2014 Jul 21-22; Singapore.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download