1. Sowman PF, Flavel SC, McShane CL, Sakuma S, Miles TS, Nordstrom MA. Asymmetric activation of motor cortex controlling human anterior digastric muscles during speech and target-directed jaw movements. J Neurophysiol. 2009; 102:159–166. PMID:
19420123.
2. Larsson SG, Lufkin RB. Anomalies of digastric muscles: CT and MR demonstration. J Comput Assist Tomogr. 1987; 11:422–425. PMID:
3571582.
3. Faltaous AA, Yetman RJ. The submental artery flap: an anatomic study. Plast Reconstr Surg. 1996; 97:56–60. PMID:
8532806.
4. Tan ST. Anterior belly of digastric muscle transfer: a useful technique in head and neck surgery. Head Neck. 2002; 24:947–954. PMID:
12369074.
5. Yamada S. Beobachtungen über den Venter anterior des Musculus digastricus mandibulae bei japanischen Erwachsenen und Foeten. Acta Anat Nippon. 1935; 8:303–347.
6. Stracker O. Die Häufigkeit interponierter Muskelkörper zwischen den vorderen Bäuchen des M. digastricus. Anat Anz. 1908; 33:117–236.
7. Zlabek K. Contribution a la connaissance des anomalies duventre anterieur du digastrique de l'Homme. Arch Anat Histol Embryol. 1933; 16:357–406.
8. Mori M. Statistics on the Musculature of the Japanese. Okajimas Folia Anat Jpn. 1964; 40:195–300. PMID:
14213705.
9. De-Ary-Pires B, Ary-Pires R, Pires-Neto MA. The human digastric muscle: patterns and variations with clinical and surgical correlations. Ann Anat. 2003; 185:471–479. PMID:
14575275.
10. Standring S, Borley NR, Collins P, Crossman AR, Gatzoulis MA, Healy JC, Johnson D, Mahadevan V, Newell RL, Wigley CB. Gray's anatomy: the anatomical basis of clinical practice. 40th ed. London: Churchill Livingstone;2008. p. 435–466.
11. Netter FH. Neck. In : Hansen JT, Benninger B, Brueckner JK, Carmichael SW, Granger NA, Tubbs RS, editors. Atlas of Human Anatomy. 5th ed. Philadelphia: Elsevier;2011. p. 26–34.
12. Kim DH, Do HJ, Kim HJ, Won SY, Choi DY, Hu KS, Choi JH, Kim HJ. Anatomic variation of the anterior belly of digastric muscle and positional relationship between the posterior belly of digastric and stylohyoid muscle. Korean J Phys Anthropol. 2010; 23:9–16.
13. Kalniev M, Krastev D, Krastev N, Vidinov K, Veltchev L, Apostolov A, Mileva M. A rare variation of the digastric muscle. Clujul Med. 2013; 86:327–329. PMID:
26527971.
14. Asami Y, Kawai K, Kanoh T, Koizumi M, Honma S, Tokiyoshi A, Kodama K. Double innervation of the anterior belly of the digastric muscle. Anat Sci Int. 2006; 81:130–133. PMID:
16800298.
15. Kawai K, Koizumi M, Honma S, Tokiyoshi A, Kodama K. Derivation of the anterior belly of the digastric muscle receiving twigs from the mylohyoid and facial nerves. Ann Anat. 2003; 185:85–90. PMID:
12597132.
16. Standring S, Borley NR, Collins P, Crossman AR, Gatzoulis MA, Healy JC, Johnson D, Mahadevan V, Newell RL, Wigley CB. Gray's anatomy: the anatomical basis of clinical practice. 40th ed. London: Churchill Livingstone;2008. p. 595–614.
17. Alagöz MS, Uysal AC, Tüccar E, Sensöz O. The vascular anatomy of the digastric muscle. J Craniofac Surg. 2004; 15:114–117. PMID:
14704576.
18. O'Rahilly R, Müller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010; 192:73–84. PMID:
20185898.
19. Sargon MF, Celik HH. An abnormal digastric muscle with three bellies. Surg Radiol Anat. 1994; 16:215–216. PMID:
7940088.
20. Sarikcioglu L, Demir S, Oguz N, Sindel M. An anomalous digastric muscle with three accessory bellies and one fibrous band. Surg Radiol Anat. 1998; 20:453–454. PMID:
9932332.
21. Holibková A, Machálek L. A report on anomalies of digastric muscle. Acta Univ Palacki Olomuc Fac Med. 1999; 142:57–59. PMID:
10743725.
22. Sargon MF, Onderoğlu S, Sürücü HS, Bayramoğlu A, Demiryürek DD, Oztürk H. Anatomic study of complex anomalies of the digastric muscle and review of the literature. Okajimas Folia Anat Jpn. 1999; 75:305–313. PMID:
10217948.
23. Peker T, Turgut HB, Anil A. Bilateral anomaly of anterior bellies of digastric muscles. Surg Radiol Anat. 2000; 22:119–121. PMID:
10959680.
24. Guelfguat M, Nurbhai N, Solounias N. Median accessory digastric muscle: radiological and surgical correlation. Clin Anat. 2001; 14:42–46. PMID:
11135397.
25. Yüksel M, Yüksel E. A case with accessory digastric muscles and its clinical importance. Ann Plast Surg. 2001; 46:351–352. PMID:
11293539.
26. Celik HH, Aldur MM, Ozdemir B, Akşit MD. Abnormal digastric muscle with unilateral quadrification of the anterior belly. Clin Anat. 2002; 15:32–34. PMID:
11835541.
27. Aktekin M, Kurtoğlu Z, Oztürk AH. A bilateral and symmetrical variation of the anterior belly of the digastric muscle. Acta Med Okayama. 2003; 57:205–207. PMID:
14627073.
28. Fujimura A, Onodera M, Feng XY, Osawa T, Nara E, Nagato S, Matsumoto Y, Sasaki N, Nozaka Y. Abnormal anterior belly of the digastric muscle: a proposal for the classification of abnormalities. Anat Sci Int. 2003; 78:185–188. PMID:
14527134.
29. Turan-Ozdemir S, Oygucu IH, Kafa IM. Bilateral abnormal anterior bellies of digastric muscles. Anat Sci Int. 2004; 79:95–97. PMID:
15218629.
30. Loukas M, Louis RG, Kapos T, Kwiatkowska M. A case of a bilateral accessory digastric muscle. Folia Morphol (Warsz). 2005; 64:233–236. PMID:
16228962.
31. Ozgursoy OB, Kucuk B. Unique variation of digastric muscle: a confusing landmark for head and neck surgeons. Acta Otolaryngol. 2006; 126:881–883. PMID:
16846934.
32. Bakirci S, Kafa IM, Uysal M, Sendemir E. Anterior belly anomalies of the digastric muscle: case report. Eur J Anat. 2007; 11:201–203.
33. Ozgur Z, Govsa F, Ozgur T. Bilateral quadrification of the anterior digastric muscles with variations of the median accessory digastric muscles. J Craniofac Surg. 2007; 18:773–775. PMID:
17667663.
34. Reyes G, Contreras C, Ramirez LM, Ballesteros LE. The digastric muscle's anterior accessory belly: case report. Med Oral Patol Oral Cir Bucal. 2007; 12:E341–E343. PMID:
17767094.
35. Mehta V, Gupta V, Arora J, Yadav Y, Suri RK, Rath G. Bilateral bipartite origin of the posterior belly of digastric muscle: a clinico-anatomical appraisal. Anatomy. 2011; 5:44–47.
36. Kyung DS, Lee JH, Lee YP, Kim DK, Choi IJ. Bilateral variations of the head of the digastric muscle in Korean: a case report. Anat Cell Biol. 2011; 44:241–243. PMID:
22025977.
37. Yamazaki Y, Shibata M, Ushiki T, Isokawa K, Sato N. Bilateral, asymmetric anomalies of the anterior bellies of digastric muscles. J Oral Sci. 2011; 53:523–527. PMID:
22167040.
38. Quadros LS, Saini H, Babu A, Kiruba L. Unilateral variant anterior belly of the digastric muscle. Int J Anat Var. 2013; 6:101–102.
39. Rani A, Chopra J, Rani A, Verma RK. Duplicated anterior belly of the digastric muscle. Singapore Med J. 2013; 54:e131–e132. PMID:
23712787.
40. Wood J. XVII. Variations in human myology observed during the winter session of 1867-68 at King's College, London. Proc R Soc Lond. 1868; 16:483–525.
41. Le Double AF. Traite des variations du systeme musculaire de homme et de leur signification au point de vue de l'anthropologie zoologique. Paris: Schleicher Freres;1897.
42. Adachi B. Beiträge zur Anatomie der Japaner. XII. Die Statistik der Muskelvarietäten. Zeitschr Morphol Anthropol. 1909; 12:261–312.
43. Liquidato BM, Barros MD, Alves AL, Pereira CS. Anatomical study of the digastric muscle: variations in the anterior belly. Int J Morphol. 2007; 25:797–800.
44. Ozgur Z, Govsa F, Celik S, Ozgur T. An unreported anatomical finding: unusual insertions of the stylohyoid and digastric muscles. Surg Radiol Anat. 2010; 32:513–517. PMID:
19763380.
45. Muraki AS, Mancuso AA, Harnsberger HR, Johnson LP, Meads GB. CT of the oropharynx, tongue base, and floor of the mouth: normal anatomy and range of variations, and applications in staging carcinoma. Radiology. 1983; 148:725–731. PMID:
6878693.
46. Yamada H. An abnormity in the venter mandibularis of M. biventer mandibulae. J Kyushu Dent Soc. 1957; 11:179–186.
47. Komatsu K, Tsuchiya H, Gunji T, Gunji K, Yoshida S, Wakita M, Kobayashi S. Two anomalies of the anterior belly of M. digastricus. Niigata Dent J. 1981; 11:1–7.
48. Sato O, Yoshida S, Kobayashi S. M. cleidohyoideus and anomaly of the anterior belly of M. digastricus. Niigata Dent J. 1986; 16:23–29.
49. Toldt C. An atlas of human anatomy for students and physicians. 7th ed. New York: Rebman Company;1907.
50. Andreo JC, Caldas Navarro JA, Toledo Filho JL. Anatomical study on the variations of the anterior belly of the digastric muscle. Rev Chil Anat. 1997; 15:111–114.
51. Mascaro MB, Picoli LC, Santos FM, Bonsi AB, Souza MR, Prosdócimi FC. Anatomical variation of the anterior belly of the digastric muscle: case report and clinical implications. J Morphol Sci. 2011; 28:72–75.
52. Sakamoto Y, Akita K. Supernumerary muscle bundles in the submental triangle: their positional relationships according to innervation. Surg Radiol Anat. 2004; 26:245–253. PMID:
14872289.
53. Spiro J, Rendell JK, Gay T. Activation and coordination patterns of the suprahyoid muscles during swallowing. Laryngoscope. 1994; 104(11 Pt 1):1376–1382. PMID:
7968167.
54. Clement WA, Graham I, Ablett M, Rawlings D, Dempster JH. Intramuscular hemangioma of the posterior belly of the digastric muscle failing to highlight on magnetic resonance imaging. Ann Otol Rhinol Laryngol. 2002; 111:1050–1053. PMID:
12450183.
55. Batra AP, Mahajan A, Gupta K. Marginal mandibular branch of the facial nerve: An anatomical study. Indian J Plast Surg. 2010; 43:60–64. PMID:
20924452.
56. Pandey D. Suspension of the tongue to the digastric tendon following resection of the anterior mandibular arch for oral cancer prevents postoperative tongue fall and avoids the need for tracheostomy. Indian J Cancer. 2012; 49:11–14. PMID:
22842162.
57. Connell BF, Shamoun JM. The significance of digastric muscle contouring for rejuvenation of the submental area of the face. Plast Reconstr Surg. 1997; 99:1586–1590. PMID:
9145126.
58. Loukas M, Thorsell A, Tubbs RS, Kapos T, Louis RG Jr, Vulis M, Hage R, Jordan R. The ansa cervicalis revisited. Folia Morphol (Warsz). 2007; 66:120–125. PMID:
17594670.
59. Mwachaka PM, Ranketi SS, Elbusaidy H, Ogeng'o J. Variations in the anatomy of ansa cervicalis. Folia Morphol (Warsz). 2010; 69:160–163. PMID:
21154286.
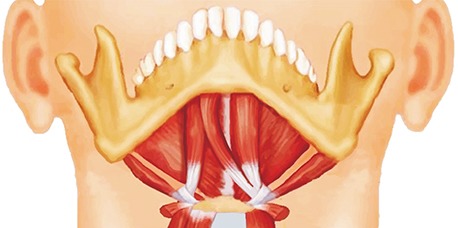
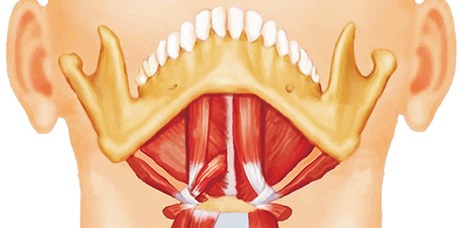
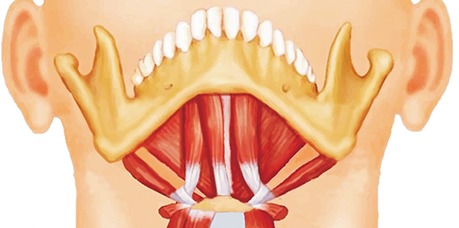
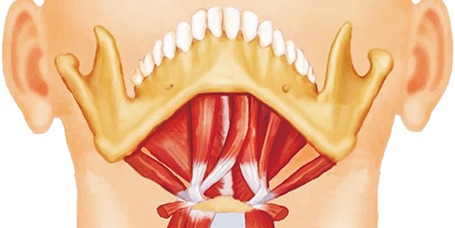




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download