Abstract
Application of magnetic resonance imaging (MRI) for assessment of pulmonary disease has been limited, due to susceptibility to cardiac pulsation, respiratory motion, and inhomogeneity of the magnetic field of the lung. With technical advances of MRI and unmet clinical needs for more accurate diagnosis and assessment of the disease, however, the use of MRI for evaluation of the lung has broadened. Herein, we present a case of pneumonic-type lung adenocarcinoma in a patient with history of anaphylactic shock to iodinated contrast medium, in which MRI played a critical role for targeted lung biopsy and cancer staging. Through this paper, we would like to report potential value of MRI in assessment of lung cancer.
Lung cancer is the leading cause of cancer-related deaths globally. As prognosis of lung cancer improves significantly with detection in early stages, timely diagnosis and accurate assessment of disease extent is critical (1). Imaging studies play a critical role in diagnosis and staging of lung cancer. Computed tomography (CT) and fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT, have been mainstays for lung cancer staging and evaluation of treatment response (234). However, it is not always capable of distinguishing lung cancer from other lung abnormalities such as pneumonia, benign hypervascular tumors, or granulomas with these modalities. In such cases, there have been several reports that magnetic resonance imaging (MRI) may provide added value in diagnosing lung cancer (45).
With recent technical advances, MRI was introduced as an emerging modality for lung imaging. Although lung MRI has many obstacles to overcome, such as extremely rapid T2* decay of lung tissue, cardiac pulsation, and respiratory motion, MRI is definitely superior to CT scan in aspects of soft tissue contrast, tissue characterization, and lack of ionizing radiation (123). In addition, MRI can be an alternative to CT, for those with hypersensitivity reactions to CT contrast medium. Herein, we present a case of pneumonic-type lung adenocarcinoma in a patient with history of anaphylactic shock to iodinated contrast medium, in which MRI played a critical role for targeted lung biopsy and cancer staging.
A 70-year-old woman, a non-smoker, was referred to a pulmonologist of our institution for prolonged cough and whitish sputum, which developed 5 months ago. Her primary-care physician diagnosed her with community acquired pneumonia and prescribed empirical antibiotics for months, but the patient's symptoms did not show any response or improvement. The patient had undergone right posterior sectionectomy of the liver for intraductal papillary mucinous neoplasm of the bile duct, 4 years prior to the visit. Her vital signs were stable, and physical examination disclosed no remarkable findings. Laboratory tests revealed mildly elevated lactate dehydrogenase (229 IU/L), and C-reactive protein level (CRP, 6.4 mg/L).
Chest radiograph was obtained, and showed a large mass-like opacity in the left upper lobe. Compared with previous chest radiographs taken at a local clinic 2 and 4 months ago respectively, abnormal opacity in the left upper lobe had been growing gradually (Fig. 1a–c). Subsequently, chest CT was performed (iCT 256, Philips Healthcare, Cleveland, OH, USA). As the patient had experienced anaphylaxis to iodinated contrast agent (iohexol) previously, CT was obtained without contrast administration. CT scan demonstrated an area of dense consolidation, and ground-glass opacity in the left upper lobe (Fig. 1d). Bronchoscopy was followed, but there was no endobronchial lesion, and tissue acquisition was not available.
For differential diagnosis of pneumonia and pneumonic-type lung cancer, MRI was performed with 3-Tesla MR system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) (Table 1). Axial half-Fourier acquisition single shot turbo spin-echo (HASTE) image showed an area of high signal intensity in the left upper lobe (Fig. 2a). Consolidation has signal intensity comparable to thoracic cerebrospinal fluid (CSF), showing so-called ‘white lung sign’ (5). Pre- and post-contrast volumetric interpolated 3D gradient-echo (VIBE) images showed an area of heterogeneous enhancement, at the anterior apical portion of consolidation (Fig. 2b, c). Axial diffusion-weighted image (DWI) obtained with a b factor of 800 s/mm2 showed an area of high signal intensity, with low apparent diffusion coefficient (ADC) value at the corresponding area (Fig. 2d, e).
CT-guided percutaneous core biopsy of the lung was performed, targeting the area showing diffusion restriction, and the pathologic result was lung adenocarcinoma (Fig. 3). Because the tumor was operable based on MRI findings, curative left upper lobectomy was performed. Final histopathologic result was invasive adenocarcinoma with gland formation, and extensive necrosis with pathologic stage of T3N0M0 (Fig. 4). The patient is currently receiving adjuvant chemotherapy with paclitaxel and cisplatin.
Our case has clinical implication, in that we proved use of lung MRI in clinical practice in several distinct aspects. First, we showed potential value of lung MRI as an alternative imaging modality of chest CT, in diagnosis and staging of lung cancer in patients with contraindication to iodinated contrast medium. Second, we demonstrated that DWI is a useful tool for differentiating malignant tumor from pneumonia, as well as planning of targeted transthoracic lung biopsy. Finally, our case demonstrated that ‘MRI white lung sign’ on T2-weighted image is indicative of fluid component within the tumor, suggesting mucinous lung cancer as well as internal necrosis of the tumor.
In 2001, Gaeta et al. (6) defined the so-called ‘MRI white lung sign’ as consolidation or mass showing signal intensity, comparable with that of static body fluids on T2-weighted images. They suggested that the sign can be observed in obstructive pneumonitis, though rare, and the sign is specific and sensitive for differentiating mucinous adenocarcinoma from pneumonia (5). Our case, however, has shown that ‘MRI white lung sign’ can represent intra-tumoral mucin, as well as area of necrosis within non-mucinous lung adenocarcinoma. There have been many studies reported about unique characteristics of invasive mucinous adenocarcinomas, showing differences from non-mucinous adenocarcinomas in terms of clinical, pathologic, genomic, and prognostic aspects (7). Thus, our case has clinical significance in that we showed ‘MRI white lung sign’ is specific for mucinous adenocarcinoma, as well as pneumonic-type non-mucinous adenocarcinoma with extensive necrosis.
With regard to pulmonary DWI, many prior studies have demonstrated that malignant lung lesions, show high signal intensity on DWI and low ADC value (234). According to a study by Guimaraes et al. (8), DWI can also facilitate planning of lung biopsy. They insisted that an adequate specimen can be acquired by targeting the area showing diffusion restriction, when the malignancy-suspected lesion is mixed with other lung pathologies, such as atelectasis, consolidation, and necrosis. We also verified effectiveness of DWI, by targeting the area showing diffusion restriction within a pneumonic-type lung cancer, for successful acquisition of an adequate tumor specimen.
For accurate staging and proper management of lung cancer, it is important to determine invasion of surrounding structures such as chest wall, heart, mediastinum, and aorta on imaging studies. Contrast enhance CT scan is a modality of choice for pre-operative cancer staging. However, as in our case, it is difficult to obtain contrast enhance CT scan in some patients, because of clinical reasons such as history of anaphylaxis to iodine contrast media. Also, there are cases that accurate determination whether the tumor invades adjacent structures or not, is impossible only with CT scan, due to inconspicuousness of margins. In such a situation, MRI may be an alternative option. Indeed, there have been many reports that MRI is superior to CT, in visualization of tumor extension to adjacent structures (123). In addition, the gadolinium agent can be used irrespective of hypersensitivity to the iodine agent (9). Too, our case also proved potential use of PET/MR as a robust technique for pre-operative staging of lung cancer, allowing for one-stop evaluation of local tumor staging on MRI and distant metastasis based on PET (10).
In summary, we present use of MRI for imaging of lung adenocarcinoma with extensive necrosis in a patient unable to undergo contrast-enhanced CT scan, due to history of anaphylactic shock to iodinated contrast medium. We showed that lung MRI could play an important role in diagnosis and staging of lung cancer and planning of targeted lung biopsy, proving potential value as an alternative modality of enhanced chest CT.
Figures and Tables
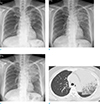 | Fig. 1Sequential chest radiographs obtained at 4 months (a), 2 months (b) prior to visit, and at the time point of the visit (c) demonstrate increasing extent of poorly-defined consolidation in the left upper lobe. High resolution axial CT (d) shows an area of dense consolidation and ground-glass opacity in the left upper lobe. |
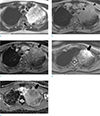 | Fig. 2(a) Axial half-Fourier acquisition single shot turbo spin-echo (HASTE) MRI shows high signal intensity consolidation in the left upper lobe. Consolidation has signal intensity comparable to cerebrospinal fluid within the thoracic spinal canal (arrow). (b, c) Pre- and post-contrast volumetric interpolated 3D gradient-echo (VIBE) images show an area of heterogeneous enhancement, at the anterior apical portion of consolidation (arrow). The fat plane between the mass and adjacent vessels were relatively well-preserved, whereas the boundary between the mass and anterior pleura was not well-delineated (arrowheads). (d, e) Axial diffusion-weighted image obtained with a b factor of 800 s/mm2 shows an area of high signal intensity, with low apparent diffusion coefficient value at the corresponding area (arrows). |
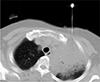 | Fig. 3CT-guided percutaneous core biopsy of the lung was performed targeting the area showing diffusion restriction. |
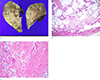 | Fig. 4(a) Resected lung tumor specimen showing ill-defined whitish solid lesion (arrows) on the cut surface. (b, c) Hematoxylin-eosin stained specimen demonstrates invasive adenocarcinoma with gland formation and extensive necrosis (magnification of × 40 and × 400, respectively). |
Table 1
Summary of Magnetic Resonance Imaging Protocol for Lung Cancer

ADC = apparent diffusion coefficients; DWI = diffusion weighted image; FA = flip angle; HASTE = half-Fourier acquisition single shot turbo spin-echo; NA = not applicable; ST = slice thickness; TE = echo time; TR = repetition time; TRUFI = true fast imaging with steady-state-free precession; VIBE = volumetric interpolated breath-hold examination
References
1. Khalil A, Majlath M, Gounant V, Hess A, Laissy JP, Debray MP. Contribution of magnetic resonance imaging in lung cancer imaging. Diagn Interv Imaging. 2016; 97:991–1002.

2. Hochhegger B, Marchiori E, Sedlaczek O, et al. MRI in lung cancer: a pictorial essay. Br J Radiol. 2011; 84:661–668.

3. Koyama H, Ohno Y, Seki S, et al. Magnetic resonance imaging for lung cancer. J Thorac Imaging. 2013; 28:138–150.

4. Kim HS, Lee KS, Ohno Y, van Beek EJ, Biederer J. PET/CT versus MRI for diagnosis, staging, and follow-up of lung cancer. J Magn Reson Imaging. 2015; 42:247–260.

5. Gaeta M, Ascenti G, Mazziotti S, Contiguglia R, Barone M, Mileto A. MRI differentiation of pneumonia-like mucinous adenocarcinoma and infectious pneumonia. Eur J Radiol. 2012; 81:3587–3591.

6. Gaeta M, Minutoli F, Ascenti G, et al. MR white lung sign: incidence and significance in pulmonary consolidations. J Comput Assist Tomogr. 2001; 25:890–896.

7. Liu Y, Zhang HL, Mei JZ, et al. Primary mucinous adenocarcinoma of the lung: a case report and review of the literature. Oncol Lett. 2017; 14:3701–3704.

8. Guimaraes MD, Marchiori E, Odisio BC, et al. Functional imaging with diffusion-weighted MRI for lung biopsy planning: initial experience. World J Surg Oncol. 2014; 12:203.

9. Natsume M, Sano H, Fukusada S, et al. Gadolinium as an alternative radiocontrast agent in patients with allergy to iodine-based contrast provide for useful diagnostic imagings and safely treatment of biliary tract diseases. Nihon Shokakibyo Gakkai Zasshi. 2013; 110:825–832.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download