Abstract
Objective
Highly sensitive haptoglobin measurement should be used in neonates because the haptoglobin concentration in neonates is lower than that of adults. The aim of this study was to establish the reference values of haptoglobin levels in the cord blood of uninfected neonates.
Methods
The cord blood of 29 preterm and 51 term babies was collected, and data from the mother and the newborn were recorded. The haptoglobin concentrations of 80 cord blood samples were simultaneously measured by enzyme-linked immunosorbent assay (ELISA; Assaypro, St Charles, MO, USA) and immunoturbidimetry assay (Roche Diagnostics, Basel, Switzerland). C-reactive protein (CRP) was also measured by immunoturbidimetry assay (Roche Diagnostics, Switzerland).
Results
Mean values of CRP and ELISA haptoglobin were not significantly different between preterm and term babies. The 2.5 percentile and 97.5 percentile values of ELISA haptoglobin concentration were as follows: 80 neonates, 0.01 mg/dL and 0.59 mg/dL; 29 preterm babies, 0.08 mg/dL and 0.18 mg/dL; and 51 term babies, 0.07 mg/dL and 0.23 mg/dL. There were no differences in ELISA haptoglobin concentration according to maternal underlying diseases, delivery method, usage of antibiotics or steroids before delivery, gestational age, gender of baby, or twin gestation.
Conclusion
A highly sensitive haptoglobin method should be used to determine the haptoglobin concentration in Korean newborns because the reference values of cord blood haptoglobin concentration in Korean newborns are less than the lower detection limit for commonly used immuno-turbidimetric haptoglobin measurement methods.
Go to : 
REFERENCES
1). Wang Y., Kinzie E., Berger FG., Lim SK., Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep. 2001. 6:379–85.

2). Carter K., Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol. 2007. 29:92–110.

3). Kristiansen M., Graversen JH., Jacobsen C., Sonne O., Hoffman HJ., Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001. 409:198–201.

4). Kanakoudi F., Drossou V., Tzimouli V., Diamanti E., Konstantinidis T., Germenis A, et al. Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin Chem. 1995. 41:605–8.

5). Kasvosve I., Speeckaert MM., Speeckaert R., Masukume G., Delanghe JR. Haptoglobin polymorphism and infection. Adv Clin Chem. 2010. 50:23–46.

6). Levy AP., Asleh R., Blum S., Levy NS., Miller-Lotan R., Kalet-Litman S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010. 12:293–304.

7). Cakmak A., Calik M., Atas A., Hirfanoglu I., Erel O. Can haptoglobin be an indicator for the early diagnosis of neonatal jaundice? J Clin Lab Anal. 2008. 22:409–14.

8). Liberatori S., Bini L., De Felice C., Magi B., Marzocchi B., Raggiaschi R, et al. Acute-phase proteins in perinatal human plasma. Electrophoresis. 1997. 18:520–6.

9). Kalil M., Badr-El-Din MK., Kassem AS. Haptoglobin level in normal infants and children. Alex Med J. 1967. 13:1.
10). Gitlin D., Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 1969. 48:1433–46.
11). Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999. 340:448–54.

13). Salmi TT. Haptoglobin levels in the plasma of newborn infants with special reference to infections. Acta Paediatr Scand Suppl. 1973. 241:1–55.
14). Mithal LB., Palac HL., Yogev R., Ernst LM., Mestan KK. Cord blood acute phase reactants predict early onset neonatal sepsis in preterm infants. PLoS One. 2017. 12:e0168677.

15). Chavez-Bueno S., Beasley JA., Goldbeck JM., Bright BC., Morton DJ., Whitby PW, et al. 'Haptoglobin concentrations in preterm and term newborns'. J Perinatol. 2011. 31:500–3.

16). Shih AW., McFarlane A., Verhovsek M. Haptoglobin testing in hemolysis: measurement and interpretation. Am J Hematol. 2014. 89:443–7.

17). Dobryszycka W. Biological functions of haptoglobin—new pieces to an old puzzle. Eur J Clin Chem Clin Biochem. 1997. 35:647–54.
18). Smithies O., Connell GE., Dixon GH. Inheritance of haptoglobin subtypes. Am J Hum Genet. 1962. 14:14–21.
19). McPherson RA. Specific protein. Henry JB, editor. editor.Clinical diagnosis and management by laboratory methods. 23rd ed.Philadelphia: WB Saunders Co;2017. p. 253–66.
20). Yang SE., Min WK., Park H., Chun S., Nah J., Kim JQ. Distribution of haptoglobin phenotypes in a Korean population, using the semi-automated PhastSystem. Ann Clin Biochem. 2000. 37(Pt 2):205–9.
Go to : 
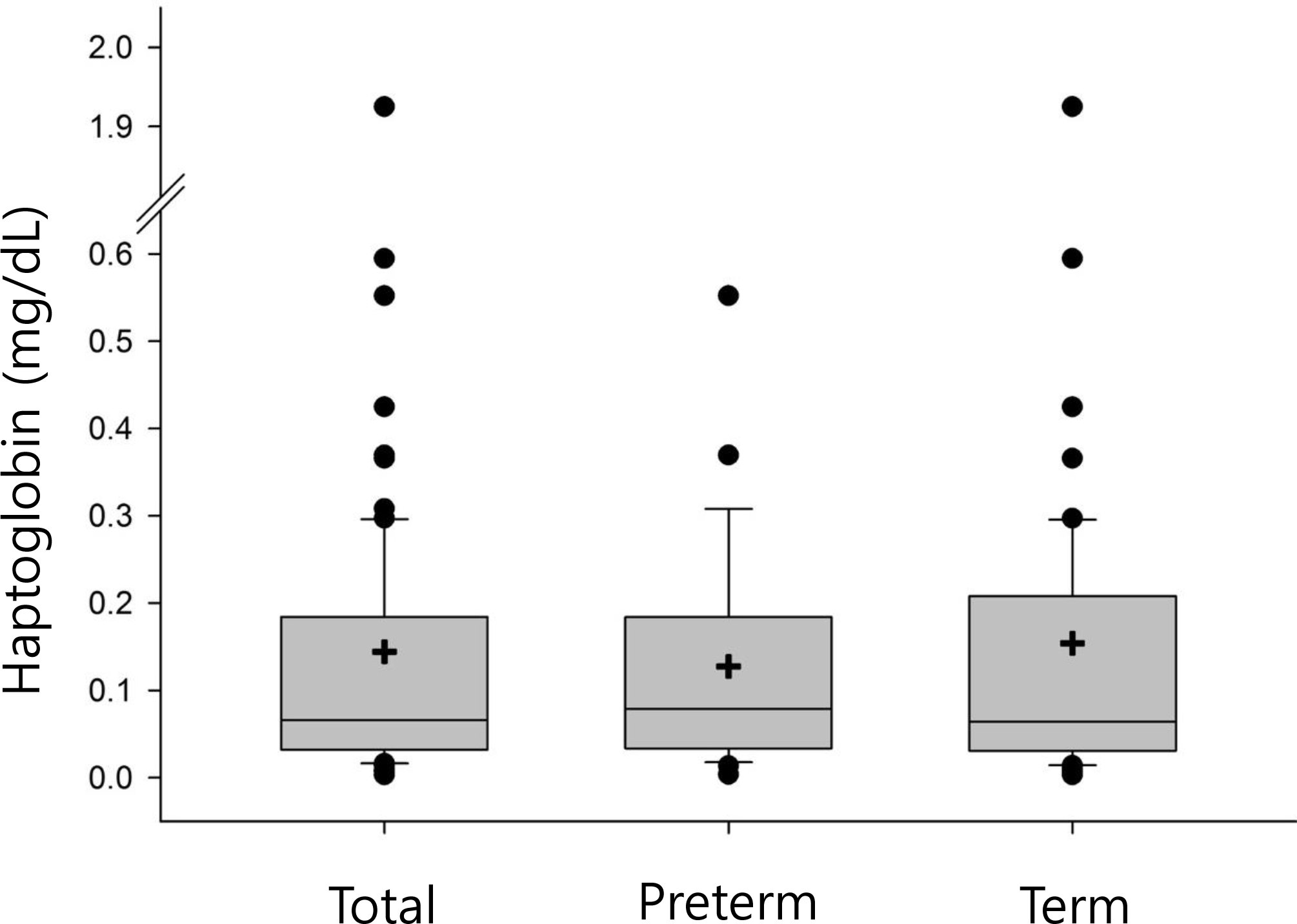 | Fig. 1.Box and whisker plot displaying the distribution of haptoglobin concentrations measured by enzyme-linked immunosorbent assay (e-haptoglobin). In the plot diagram, the central rectangle is the interquartile range, the whiskers above and below the box show the locations of the minimum and maximum excluding outliers, the line within the box marks the median, and + symbol means the mean of e-haptoglobin. |
Table 1.
Demographic Characteristics of 80 Newborns
Table 2.
Haptoglobin Concentration According to Demographic Characteristics of 80 Newborns
| Demographic characteristics | Haptoglobin concentration (mg/dL) by measurement method | ||||
|---|---|---|---|---|---|
| ITM | ELISA | P-value by Kolmogorov-Smirnov test∗ | P-value of difference | ||
| Mean±SD | Percentile (2.5-97.5%) | ||||
| Gestational week | 0.806 | ||||
| Preterm | <10† | 0.13±0.13 | 0.08-0.18 | 0.038 | |
| Term | <10 | 0.15±0.28 | 0.07-0.23 | <0.001 | |
| Gender | 0.176 | ||||
| Male | <10 | 0.18±0.30 | 0.08-0.27 | <0.001 | |
| Female | <10 | 0.11±0.13 | 0.07-0.15 | <0.001 | |
| Delivery mode | 0.140 | ||||
| Cesarean section | <10 | 0.16±0.27 | 0.09-0.23 | <0.001 | |
| Vaginal | <10 | 0.10±0.14 | 0.05-0.16 | <0.001 | |
| Rupture of amniotic membrane | 0.135 | ||||
| No spontaneous | <10 | 0.17±0.28 | 0.09-0.25 | <0.001 | |
| Spontaneous | <10 | 0.10±0.13 | 0.05-0.15 | <0.001 | |
| Number of fetus | 0.936 | ||||
| Singleton | <10 | 0.15±0.25 | 0.09-0.21 | <0.001 | |
| Twin | <10 | 0.11±0.09 | 0.05-0.17 | 0.200 | |
| Maternal chronic disease | 0.325 | ||||
| No | <10 | 0.11±0.11 | 0.08-0.14 | <0.001 | |
| Yes | <10 | 0.22±0.40 | 0.05-0.40 | <0.001 | |
| Maternal usage of antibiotics | 0.292 | ||||
| No | <10 | 0.15±0.31 | 0.05-0.25 | <0.001 | |
| Yes | <10 | 0.14±0.13 | 0.09-0.18 | 0.004 | |
| Maternal usage of steroids | 0.594 | ||||
| No | <10 | 0.16±0.27 | 0.09-0.23 | <0.001 | |
| Yes | <10 | 0.10±0.09 | 0.06-0.14 | 0.010 | |
| Total | <10 | 0.14±0.24 | 0.01-0.59 | <0.001 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download