Introduction
Staphylococcus lugdunensis is a coagulase-negative staphylococcus (CoNS) that is a part of the normal human skin flora. Even though it belongs to CoNS family, it is known to cause more severe infections in a similar fashion to
Staphylococcus aureus [
123]. Therefore, isolation of this pathogen on culture should be considered as a true pathogen, rather than a contaminant, especially if isolated from sterile site cultures. It is a known etiology of skin and soft tissue infections [
4], bacteremia and native valve endocarditis [
15]. Other sites of infections caused by
S. lugdunensis include bone and prosthetic joint [
6], catheter and other device-associated infections, as well as the central nervous system [
27]. It is unusual to cause urinary tract infection [
28].
The available data on the diagnosis and clinical characteristics of
S. lugdunensis infection in patients with underlying malignancy is limited. Nesher et al. recently published a retrospective review of 45 cases from a comprehensive cancer center and studied the characteristics of
S. lugdunensis infections in cancer patients[
9]. Skin and soft tissue infections were the most common entities; a significant number of patients had a history of surgical procedures or an implanted medical device [
9]. Noguchi et al. made an interesting observation that group D clones of
S. lugdunensis may be associated with colon carcinoma [
10]. However,
S. lugdunensis is not well studied in cancer populations.
The objective of this study was to better understand and recognize the infection caused by S. lugdunensis in cancer patients.
Materials and Methods
We retrospectively reviewed all patients with cultures positive for S. lugdunensis between January 2000 and January 2018 using the H. Lee Moffitt Cancer Center and Research Institute institutional database system. Inclusion criteria for chart review were adults above age 18 who had a positive culture (blood, urine, wound, abscess, cerebrospinal fluid) for S. lugdunensis. All culture samples were analyzed in the microbiology laboratory at the Moffitt Cancer Center and organism identification was performed through standardized microbiological testing methods: first, we perform coagulase test which will be negative, then we use VITEK2 machine with the GP ID Card (BioMerieux, Durham, NC, USA). An automated culture method was used to confirm the identification and susceptibilities of S. lugdunensis. General patient characteristics including age, gender, and underlying cancer diagnosis were included in the analysis. Patient cases were classified into either having a true S. lugdunensis infection versus a colonization/contamination when positive culture for S. lugdunensis was not considered to be clinically significant. For body fluid aspirates or wound cultures positive for S. lugdunensis, a true infection was diagnosed when local (e.g., erythema, wound drainage) and/or systemic symptoms (e.g., fever, leukocytosis) were present in addition to a positive culture. For urine cultures positive for S. lugdunensis, a true infection was diagnosed when urinary symptoms (e.g., frequency or burning with urination, hematuria) were present in addition to a positive culture. A true infection of the bloodstream or central nervous system was suspected only when clinically relevant symptoms were present in addition to a positive culture. Informed consent was waived due to the retrospective nature of this work and de-identified chart review. The methods of this study were approved by the University of South Florida Institutional Review Board (Approval #: Pro00034756) and Moffitt Cancer Center Scientific Review Committee. Quantitative description was used for summarizing the data. Microsoft Excel (Sacramento, CA, USA) was used to summarize data in mean (± standard deviation) or number (percentage).
Results
A total of 24 patients had a positive culture for
S. lugdunensis at the H. Lee Moffitt Cancer Center and Research Institute within the study period. General characteristics of the patients are shown in
Table 1. The ages of the patients ranged between 29 to 79 years (Mean age 60.9 ± 12.4 years). Thirteen patients were male and eleven patients were female. Underlying malignancies were also analyzed: 20 patients (83.3%) had solid malignancies, and 4 patients (16.7%) had hematologic malignancy such as acute myelogenous leukemia, chronic myelogenous leukemia and plasma cell leukemia. Among 20 patients with solid malignancies, 8 patients (37.5%) had urogenital cancers (three renal cell carcinomas among others), 7 patients (33.3%) had skin cancers (squamous cell carcinoma and melanoma), and 5 patients had other cancers such as colorectal, breast, or lung cancer (
Table 1). None of the patients had neutropenia defined as an absolute neutrophil count less than 500 neutrophils per microliter of blood.
Table 1
General characteristics (age, gender, type of malignancy) of the patients included in the case series

|
All patients (N = 24) |
True infection group (N = 14) |
Colonization group (N = 10) |
|
Age, mean ± SD, years |
60.9 ± 12.4 |
60.7 ± 14 |
61.1 ± 8.1 |
|
Gender, No. |
|
|
|
|
Male |
13 |
8 |
5 |
|
Female |
11 |
6 |
5 |
|
Underlying malignancy |
|
|
|
|
Solid tumors, No. |
20 |
12 |
8 |
|
|
Urogenital cancer |
8 |
5 |
3 |
|
|
Skin cancer |
7 |
3 |
4 |
|
|
Othera
|
5 |
4 |
1 |
|
Hematologic malignancy, No. |
4 |
2 |
2 |

The source of cultures was analyzed (
Table 2). Culture specimens were obtained from a body fluid aspirate or a wound in 12 patients (50%), 7 (29.2%) urine, four (16.7%) blood, and one cerebrospinal fluid. Based on clinical manifestation, 14 of the 24 patients (58.3%) were treated as a true infection, and the remainder as a contamination or colonization (10 out of 24 patients; 41.7%). In the group of patients diagnosed with a true infection, seven out of 14 (50%) were diagnosed with SSTI, one with a pelvic abscess (7%), one patient (7%) had a catheter-associated bloodstream infection, and five patients (35.7%) had a UTI. In patients diagnosed with a true infection, three cultures were polymicrobial. Two urine cultures grew
S. lugdunensis greater than 100,000 colonies per milliliter and lower than 10,000 colonies per milliliter of mixed bacterial flora. An isolated blood culture from one of the two patients with bacteremia was also polymicrobial and grew
S. lugdunensis,
S. epidermidis, and
Bacillus species not
anthracis.
Table 2
Culture source in all patients and in the patients diagnosed with true infection

|
No. (%) |
|
Number of patients |
True infection |
|
No. of patients |
24 (100) |
14 (62.5) |
|
Blood, No. (%) |
4 (16.7) |
2 (14.3) |
|
Urine, No. (%) |
7 (29.2) |
5 (35.7) |
|
Wound or body fluid, No. (%) |
12 (50.0) |
7 (50) |
|
CSF, No. (%) |
1 (4.2) |
0 (0) |

Characteristics of the patients that were diagnosed with a true infection versus colonization group are described in
Table 3 and
Table 4 respectively.
Table 3
Characteristics of the patients diagnosed with a true infection

|
Case |
Gender |
Age |
Underlying Malignancy |
Specimen |
Diagnosis/Possible Source |
Presentation/Clinical Symptoms |
Prior Procedures |
CVC or Other Device |
Time to Diagnosis of Infectiona
|
Antibiotic Treatment |
Other Treatment Intervention |
|
1 |
M |
55 |
Urothelial cancer |
Wound culture (deep abdominal wound) |
Abdominal-pelvic subcutaneous abscess |
Fever, chills, abdominal pain |
Repair of large ventral hernia with primary closure |
No |
28 days |
Cephalexin for 14 days |
I&D of abdominal abscesses, Wound Vacuum placement |
|
2 |
F |
68 |
Poorly differentiated chordoma of the foot |
Superficial wound culture |
Below-knee amputation site soft tissue infection and fluid collection |
Pain and burning at prior amputation site |
Right below knee amputation |
No |
8 months and 26 days |
Doxycycline and Ciprofloxacin for 14 days |
none |
|
3 |
M |
67 |
Prostate cancer |
Body fluid aspirate culture |
Pelvic abscess |
Fever, chills |
Robotic radical prostatectomy |
No |
2 months and 14 days |
Linezolid for 14 days, followed by Sulfamethoxazole/Trimethoprim for 28 days |
CT-guided drainage of pelvic fluid collection |
|
4 |
M |
29 |
Dermatofibrosarcoma |
Body fluid aspirate culture |
Left groin fluid collection at prior surgical site |
Left groin pain |
Inguinal mass resection |
No |
23 days |
Amoxicillin/Clavulanate for 14 days |
US-guided fluid collection aspiration |
|
5 |
M |
73 |
Metastatic penile cancer |
Body fluid aspirate culture |
Left thigh fluid collection |
Left thigh erythema and pain |
Total urethrectomy, peritoneal urethrostomy, bilateral groin lymph node dissection |
Port |
1 month and 29 days |
Cephalexin for 28 days |
US-guided drainage |
|
6 |
M |
47 |
Melanoma of the thigh |
Body fluid aspirate culture |
Left thigh abscess |
Fever, pain at abscess site |
Left thigh melanoma excision with groin LN dissection |
No |
2 months and 11 days |
Vancomycin and Ampicillin/Sulbactam for 3 days, transitioned to oral doxycycline on discharge for 14 more days |
CT-guided drainage of abscess |
|
7 |
F |
41 |
Melanoma of the buttock |
Body fluid culture (JP drain) |
Groin surgical site infection (wound infection/cellulitis) |
Fever, Purulent drainage from wound |
Melanoma site resection and right groin lymphadenectomy |
No |
31 days |
Amoxicillin/Clavulanate and ciprofloxacin for 10 days |
JP drainage |
|
8 |
M |
64 |
AML, s/p allogeneic HSCT |
Blood culture (one culture positive) |
Bacteremia, possibly CVC-associated |
Fatigue, leukopenia |
None |
Yes (Hickman catheter) |
N/A |
Vancomycin IV for 10 days |
CVC removal |
|
9 |
F |
78 |
Renal cell carcinoma |
Blood Culture (one culture positive, polymicrobial) |
Bacteremia, from prior surgical wound infection/dehiscence |
Wound dehiscence |
Spinal instrumentation and fusion |
No |
1 month and 18 days |
Vancomycin IV and oral Ciprofloxacin for 42 days |
Spinal hardware removal |
|
10 |
M |
49 |
Germ cell testicular cancer |
Urine culture |
UTI |
Dysuria, UA pyuria WBC >100/hpf, abdominal pain |
None |
Left ureteral stent |
3 months and 27 days |
Ciprofloxacin for 14 days |
none |
|
11 |
M |
64 |
Renal cell carcinoma |
Urine culture |
UTI |
Urinary retention, UA pyuria WBC >100/hpf |
Left open radical nephrectomy |
Intermittent straight catheterizations |
26 days |
Ciprofloxacin for 10 days |
none |
|
12 |
F |
59 |
Metastatic breast cancer |
Urine culture |
UTI |
UTI symptoms, not other specified, UA pyuria WBC >100/hpf |
none |
Implantable port |
N/A |
Nitrofurantoin for 14 days |
none |
|
13 |
F |
77 |
Metastatic colorectal cancer |
Urine culture |
UTI |
Urinary frequency, pain with urination, UA WBC >100/hpf |
none |
none |
N/A |
Trimethoprim-sulfamethoxazole for 14 days |
none |
|
14 |
F |
79 |
Chronic lymphocytic leukemia |
Urine culture |
UTI |
Frequent urination, UA WBC >100/hpf |
none |
none |
N/A |
Ciprofloxacin for 7 days |
none |

Table 4
Characteristics of the patients diagnosed with a colonization or contamination

|
Case |
Gender |
Age |
Underlying malignancy |
Specimen |
Diagnosis/Possible Source |
Presentation/Clinical Symptoms |
Prior Procedures |
CVC or Other Device |
Antibiotic Treatment |
Other Treatment Intervention |
|
1 |
F |
60 |
Squamous cell carcinoma of skin/hand |
Wound |
Colonization |
Chronic non-healing radiation ulcer |
Surgical resection of radiation ulcer |
None |
Not treated |
None |
|
2 |
M |
43 |
Metastatic melanoma |
CSF |
Contamination |
None |
Intrathecal cytarabine injection |
Ommaya reservoir |
Not treated |
None |
|
3 |
M |
59 |
Chronic lymphocytic leukemia |
Wound |
Colonization |
Non-healing scalp wound |
Wound debridement, scalp flap |
Port |
Cephalexin given post-operatively after flap placed; wound was not suspected to be infected |
None |
|
4 |
M |
69 |
Bladder cancer |
Urine |
Colonization |
None |
Cystourethroscopy |
None |
Prophylactic Bactrim |
None |
|
5 |
F |
65 |
Squamous cell carcinoma of skin |
Wound |
Colonization |
Painful nodule |
Recent excision of cancer |
None |
Not treated |
None |
|
6 |
F |
58 |
Squamous cell carcinoma of skin |
Wound |
Colonization |
Pain and erythema |
Recent excision of cancer |
None |
Not treated |
None |
|
7 |
F |
72 |
Cervical cancer |
Urine |
Colonization |
Hematuria |
None |
None |
Not treated |
None |
|
8 |
M |
58 |
Squamous cell carcinoma of lung |
Wound |
Colonization |
Erythema of skin |
None |
None |
Not treated |
None |
|
9 |
M |
68 |
Renal cell carcinoma |
Blood |
Colonization |
Hypotension |
None |
None |
Vancomycin initially, then discontinued as culture was felt to be contaminant, hypotension was attributed to volume depletion |
None |
|
10 |
F |
59 |
Plasma cell leukemia |
Blood |
Colonization |
Fever, diarrhea, vomiting |
None |
None |
Vancomycin initially, then discontinued as symptoms were attributed to other diagnosis |
None |

Out of 14 patients that had a culture positive for S. lugdunensis, seven (50%) were diagnosed with skin and soft tissue infection (SSTI) based on a positive wound or fluid aspirate culture and clinical presentation in six patients. One of the seven patients diagnosed with SSTI had polymicrobial bacteremia as described above. Polymicrobial nature of the isolated blood culture raised a possibility of a contamination. However, the patient had a dehisced wound with infected hardware near the thoracic spine, which was thought to be a potential source of patient's bacteremia. Among patients with SSTI, two patients had infection at the groin site, two in the thigh area, one in the abdominal wall, one at the prior thoracic region surgical site, and one patient had an infection at a prior below the knee amputation site. Of note, all seven patients with SSTIs had a prior history of surgery, such as a tumor resection, lymph node dissection, ventral hernia repair, or kyphoplasty. The most common presentation for patients with SSTI was pain at the surgical site in five patients, fever in three patients, purulent drainage from the wound in one patient, erythema at the surgical site in one patient, and wound dehiscence in one patient. All seven patients were treated with antibiotics as described below; in addition, image-guided aspiration or drainage was utilized in five out of seven patients. All patients improved after the treatment.
One patient was diagnosed with a deep pelvic abscess based on the clinical presentation and a positive intraoperative deep wound culture. This patient had a history of a prior pelvic surgery and underwent image-guided fluid drainage as described in
Table 3. He was treated with antibiotics and subsequently improved.
Four patients out of 24 in our study had blood culture samples positive for S. lugdunensis. One patient had an intra-vascular device in place and was diagnosed with the catheter-associated blood-stream infection. In the second patient (as described above), the likely source of bacteremia was a spinal wound infection with infected hardware, therefore, this patient was included in the subgroup of patients diagnosed with SSTI. Based on their chart review, neither one of them was diagnosed with endocarditis, however, only the second patient had an echocardiography performed at the time of the diagnosis. In that particular patient, a transthoracic echocardiography was performed four days after bacteremia onset and was negative for any valvular changes to suggest a diagnosis of endocarditis. The above two patients were treated with intravenous antibiotics and improved. In the two remaining cases, blood culture contamination was diagnosed and treatment was not prescribed due to the lack of evidence of a clinically significant infection. Bacteremia did not re-occur in any of the patients.
Seven patients had positive urine culture for
S. lugdunensis in a significant quantity, greater than 100,000 colonies per milliliter. Five patients were diagnosed with a true infection based on the presence of symptoms such as dysuria. As described earlier, two out of five patients with UTI had polymicrobial urine culture with
S. lugdunensis isolated in a significant range greater than 100,000 colonies per milliliter; mixed bacterial flora was present in a non-significant range less than 10,000 colonies per milliliter. All five patients had significant pyuria on urinalysis, defined as a presence of 100 or more white blood cells (WBC) per high power field (hpf). One patient had a ureteral stent in place and one patient used intermittent straight catheterizations on a regular basis. All five patients received antibiotic treatment as indicated in
Table 3 and all five improved. There were two patients diagnosed with asymptomatic
S. lugdunensis bacteriuria. One of those two patients received antibiotics (trimethoprime/sulfamethoxazole [TMP/SMX]) in view of a scheduled urologic procedure.
All 14 patients that were diagnosed with a true infection were treated with antibiotics (
Table 3). One patient diagnosed with asymptomatic
S. lugdunensis bacteriuria was treated in view of an upcoming invasive urologic procedure. The most common regimens included ciprofloxacin in 3 (21%) patients, followed by cephalexin in 2 patients (14.3%). Other antimicrobials such as linezolid, amoxicillin/clavulanate, sulfamethoxazole/trimethoprim, nitrofurantoin as well as combination therapy was used as indicated in
Table 3. The duration of therapy varied from one week to 6 weeks. Both patients with bacteremia were treated with intravenous therapy. All patient had resolution of their infectious process.
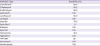
Susceptibility data confirmed that
S. lugdunensis was susceptible to most of the antibiotics except for penicillin G, which showed 100% resistance in our patient population (
Table 5). Susceptibility to oxacillin was 86.96%. For the antibiotics gentamicin, linezolid, rifampin, synercid, tigecycline, vancomycin and nitrofurantoin,
S. lugdunensis showed 100% susceptibility. To other antibiotics,
S. lugdunensis showed 80- 90% susceptibility as indicated in
Table 4.
Table 5
Antibiotic susceptibility data
|
Antibiotic Type |
Sensitivity (%) |
|
Ciprofloxacin |
90.9 |
|
Clindamycin |
95.2 |
|
Erythromycin |
90.9 |
|
Gentamicin |
100 |
|
Levofloxacin |
90.9 |
|
Linezolid |
100 |
|
Oxacillin |
87.0 |
|
Penicillin G |
0 |
|
Rifampin |
100 |
|
Synercid |
100 |
|
Tetracycline |
95.2 |
|
Tigecycline |
100 |
|
TMP-SMX |
80 |
|
Vancomycin |
100 |
|
Nitrofurantoin |
100 |

Discussion
S. lugdunensis is a Gram-positive, catalase-positive, CoNS that is a part of the normal skin flora [
7]. It most commonly colonizes the perineal and groin areas of the body [
7]. Although
S. lugdunensis is part of CoNS group, it is considered virulent and can cause destructive disease, therefore, isolation of this organism in culture should be taken seriously. Immunocompromised patients may be more vulnerable; however, limited data is available on
S. lugdunensis infections in cancer patients. We performed a retrospective chart review of the patients with positive cultures for this organism. True infection was diagnosed in 14 out of 24 patients (58.3%). Of these, 11 cultures were monomicrobial and positive only for
S. lugdunensis, and three cultures were polymicrobial as described above.
In our case series, skin and soft tissue infection was common and diagnosed in 7 patients (50%) with clinically significant isolates for
S. lugdunensis. This is consistent with prior studies indicating that
S. lugdunensis is a common pathogen of SSTI in the general population [
41112]. A recent study characterizing
S. lugdunensis infections in cancer patients from MD Anderson Cancer Center Institute found skin and soft tissue infection in 80% of the cases [
9]. Of note, all of these patients had recent surgery or an invasive intervention in the area where infection developed.
Four patients out of 24 in our study had blood culture samples positive for
S. lugdunensis. Clinically significant bacteremia was established in 2 patients and was treated; blood contamination with
S. lugdunensis was diagnosed in the other two patients. Even though
S. lugdunensis is a part of the normal skin flora and its isolation in blood cultures can represent contamination, the detection of this organism even in a single blood culture should be thoroughly evaluated as
S. lugdunensis bacteremia can be associated with an aggressive infection such as endocarditis [
31314]. A small study by Fadel et al. found that clinically significant bacteremia occurred in 16 out of 29 (45%) patients with single
S. lugdunensis-positive blood cultures [
15]. None of the patients in their study experienced bacteremia relapse; no cases of endocarditis were reported either [
15]. The study by Zinkernagel et al. reported a 50% incidence of infective endocarditis in patients with S.
lugdunensis bacteremia; all of the cases with endocarditis were community-acquired [
13]. Most of the other cases of clinically significant
S. lugdunensis bacteremia, especially if health-care associated, were known to be associated with central venous catheters [
131617]. Of note, our study had very few patients with underlying hematological malignancies (16.7%), whereas most had a solid malignancy as an underlying diagnosis. This could be explained by a common use of prophylactic antibiotics such as fluoroquinolones and TMP/SMX in patients with hematologic malignancies and neutropenia. Both fluoroquinolones and TMP/SMX have high activity against
S. lugdunensis, potentially preventing the majority of infections caused by this organism but not by other CoNS species in this particular patient population.
In our study, 7 patients were found to have urine cultures positive for
S. lugdunensis. Five out of seven patients were diagnosed with UTI and were treated with antibiotics. In addition, one out of two patients in the colonized group received antibiotics due to an upcoming invasive urologic procedure. Our study shows a much higher rate of diagnosis of
S. lugdunensis-associated UTI compared to other case series. In the study by Haile et al., only 9 out of 30 patients (30%) with a urine culture positive for
S. lugdunensis were thought to be clinically significant and treated for UTI [
8]. A recent article by Nesher et al. characterizing
S. lugdunensis infections in cancer patients at the MD Anderson Cancer Institute reported a very low rate of
S. lugdunensis genito-urinary infections in only 2 out of 45 patients [
9]. Based on the prior literature review,
S. lugdunensis is considered an infrequent cause of UTI, despite its common colonization of the groin and perineum.
Prior studies confirm that
S. lugdunensis remains susceptible to a variety of antimicrobial agents [
12]. In our study, resistance to penicillin G was universal at 100% and about 10% of all the isolates were resistant to oxacillin. A study by Kleiner et al. reported only 1 out of 35 isolates (3%) being resistant to oxacillin [
11]. Another study of Tan et al., reported only 5% resistance to oxacillin in 106 analyzed isolated [
18]. Penicillin and oxacillin resistance in our case series appear to be higher than has been reported in the literature, likely reflecting prior antibiotic exposure in our specific patient population.
Our case series indicate that, similar to the general population, S. lugdunensis most commonly causes skin and soft tissue infections in cancer patients, but unlike prior studies we saw more patients with UTIs. The incidence of clinically significant bacteremia was low with no cases of endocarditis in our study. We observed that S. lugdunensis remains susceptible to a variety of antibiotics, with all isolates susceptible to vancomycin and linezolid and most remain susceptible to fluoroquinolone and TMP/SMX. The limitation of our study is its retrospective nature and small sample size. In conclusion, S. lugdunensis, an organism that belongs to CoNS group, has a tendency to cause clinically significant and even aggressive infections, compared to other CoNS species. However, none of our patients had life-threatening infections and all recovered with appropriate antibiotic therapy.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download