Abstract
Purpose
Many patients with cytology proven node-positive breast cancer receive a neoadjuvant chemotherapy (NAC) treatment. We developed a nomogram to predict the breast and axillary pathologic complete responses (pCR) in patients with a cytologically proven axillary node positive breast cancer with NAC.
Methods
We selected 995 patients who were diagnosed with an invasive breast cancer and axillary lymph nodes metastasis, and who were treated with NAC followed by a curative surgery at the Samsung Medical Center between January 2007 and December 2014. The baseline patient and tumor characteristics, chemotherapy regimen, and tumor and nodal responses were thoroughly analyzed and reviewed. A nomogram was developed using a binary logistic regression model with a cross validation.
Results
Axillary pCR was achieved in 47.3% and breast pCR was achieved in 24.3% of the patients after NAC. In this case, the both pCR was associated with an initial clinical tumor stage, negative progesterone receptor status, positive human epidermal growth factor receptor 2 status, and clinical radiologic nodal responses. A nomogram was developed based on the clinical and statistically significant predictors. It had good discrimination performance (area under the curve [AUC], 0.868; 95% confidence interval, 0.84–0.89) and calibration fit as noted in that case. The cross validation had an average AUC 0.853 (0.837–0.869).
The use of neoadjuvant chemotherapy (NAC) is increasingly used for patients with operable breast cancer to allow for the use of more minimal surgery [12]. To avoid axillary lymph node dissection (ALND) or mastectomy, it is preferable to have a sentinel lymph node (SLN) surgical procedure or breast conserving surgery (BCS), which results in fewer instances of problems and lower morbidity [1345].
It should be noted that although about 30% patients had no residual disease in the axilla after NAC, ALND has been recommended for most patients with a biopsy proven node-positive breast cancer regardless of their response to NAC treatment [67]. In this case, some evidence has suggested that the nodal stage after NAC reflects the prognosis more accurately than the initial axillary status [8]. Therefore, the ALND may not be needed for patients with a complete response (CR). These patients are able to avoid postoperative morbidities such as lymphedema, arm pain, and reduced arm movement [910].
If we can predict which patients will develop axillary pathologic complete response (pCR), we can prevent ALND and if we can predict the breast pCR, we can prevent mastectomy. Additionally, both axillary and breast pCRs after a course of NAC are associated with improved oncologic survival [11]. Therefore we investigated the factors that predicted both pCRs and established a nomogram calculating the probability of both pCRs in patients who received NAC.
This study is a registered medical record review based on a prospectively collected database. We selected 995 patients who were diagnosed with an invasive breast cancer and axillary lymph nodes metastasis by ultrasound of axilla, and who were treated with NAC followed by curative surgery at Samsung Medical Center between the timeframe of January 2006 and December 2015. Patients were eligible if they met the following criteria: (1) diagnosed with an enlarged axillary lymph node (LN) by the use of a breast ultrasonography and by fine needle aspiration cytology upon an initial examination, (2) completed all cycles of the planned-dosage NAC, and (3) who had undergone a radical excision of a primary tumor and SLNB or ALND. Likewise, patients with bilateral breast cancer, previous ipsilateral axillary surgery, inflammatory breast cancer, or distant metastasis were excluded from the study.
Most patients (95.1%) received anthracycline- and/or taxane-based regimens. These regimens included anthracycline plus cyclophosphamide, followed by anthracycline-based, taxane-based, or trastuzumab regimens. The adjuvant radiotherapy was performed with tangential fields in all patients following by a BCS.
For the most part, the clinical response to treatment was evaluated by a breast magnetic resonance imaging (MRI) or a breast ultrasonography. In case of discordance between ultrasonography and MRI, we used the results of the MRI. Clinical CR of the breast was defined as a disappearance of all of the tumor deposits on a MRI or breast ultrasonography. We used RECIST (response evaluation criteria in solid tumor) criteria for clinical response evaluation. The quantification of response by using the categories of CR, partial response (PR), stable disease, and progressive disease (PD) served as a gross estimate of the chemosensitivity. And clinical nodal response was divided the categories to breast MRI or breast sonography into disappeared, decreased or no changed/increased.
A sentinel node biopsy was performed with technetium-99m sulfur colloid diluted in normal saline solution and/or vital blue dye (0.8% indigo carmine). The site and timing of the agent administration were at the physician's discretion: the radiolabeled colloid was injected 2 to 6 hours before the scheduled surgery, and/or 5 mL of 0.8% indigo carmine was injected periareolarly, and the breast was massaged for 5 minutes. For the sulfur-colloid injection, a handheld gamma detection probe was used to scan the axilla transcutaneously, and was used to identify the most radioactive area. All radioactive and/or blue LNs and palpable LNs were excised and submitted as SLNs.
We measured, froze, and serially sectioned the excised SLNs transversely into 16 or 24 slices. After pathological evaluation of the sections, we fixed the remaining tissue in 10% formalin, embedded it in paraffin blocks, and finally prepared the hematoxylin and eosin (H&E)-stained sections. We designated metastatic foci of 0.2 mm to 2 mm as micrometastases, and metastatic clusters smaller than 0.2 mm were considered isolated tumor cells, whether detected by the H&E or by the use of immunohistochemistry. All of the patients underwent a breast and axillary surgery within 6 weeks of completing the NAC. The type of breast surgery was selected according to the preferences of the surgeon and the patient.
We built a nomogram based on a binary logistic regression model with the significant and predefined predictors. The model was then used to predict the probabilities of the individual patients achieving both axillary and breast pCRs to NAC. The performance of the model was quantified with respect to significant factors related to discrimination and calibration. The intolerant abilities of the model were assessed by measuring the area under the receiver-operating characteristics curve. In this case, the calibration is the agreement between the frequencies of the observed outcomes, and the probabilities predicted by the model. The calibration plot was evaluated using the Hosmer-Lemeshow goodness-of-fit test and visualized by accompanying plots. A nomogram was developed using a binary logistic regression model with a cross validation model. The cross validation has a 5-fold cross-validation model with a random split analysis in a cohort of patients. The differences were assumed to be significant when the P-value was less than 0.05. This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea (approval number: 2017-09-051). Informed consent was waived because of the low risk posed by this investigation.
In this study, the median age at the time of surgery was 45.8 years in age. The demographic, clinicopathological, and treatment characteristics of the patients included in this study are listed in Table 1. The axillary pCR was achieved in 47.3% (n = 472) of the patients who underwent axillary surgery after NAC. The breast pCR was achieved in 24.3% (n = 242) of the patients who underwent breast surgery. Generally speaking, both breast and axillary pCRs were achieved in 20.5% (n = 204) of the patients who underwent surgery. The patients with both pCRs tended to be estrogen receptor (ER) negative, progesterone receptor (PR) negative, human epidermal growth factor receptor 2 (HER2) positive, with high Ki-67, good tumor response, good nodal response and early clinical tumor stage as compared with the patients with a non-pCR.
The both pCRs were associated with an initial clinical tumor stage, negative ER status, negative PR status, positive HER2 status, high Ki-67 (Ki-67 > 20) and clinical radiologic nodal responses in a univariate analysis. The multivariate logistic regression analysis showed that the both pCRs were associated with initial clinical tumor stage, negative PR status, positive HER2 status, and clinical radiologic nodal responses (Table 2).
We made a nomogram based on the clinically and statistically significant variables noted in this study. The significant variables that can be assessed in preoperative evaluations in the multivariate analysis were the noted years of a clinical tumor stage (cT1, cT2, cT3, or cT4), PR status (negative or positive), HER2 status (negative or positive), and the clinical radiologic nodal stage (disappeared, decreased or no changed/increased) (Table 3). In this case, Fig. 1 illustrates the nomogram to calculate the probability of achieving both pCR. The total nomogram score is calculated by summing up the scores for each of the variables. In that instance, the total score can then be used to assign a probability of achieving both pCRs to individual patients using the scale.
A nomogram was developed based on the clinical and statistically significant predictors. It had good discrimination performance (AUC, 0.868; 95% CI, 0.845–0.891) and calibration fit. The calibration plot showed a good and satisfactory agreement between the predicted and observed probabilities according to an administered Hosmer-Lemeshow test (Fig. 2). We had 5-fold cross-validation model with a random split analysis in a cohort of patients and Cross validation who had an average of AUC 0.853 (0.837–0.869) (Fig. 3).
Predicting pCR after NAC in patients with breast cancer and cytologically proven nodal metastasis is important for understanding and improving the patient outcomes as well as for identifying patients in whom ALND and mastectomy might be omitted as a treatment option. We performed a registered medical record review based on a prospectively collected database and made a prediction model based on the clinicopathologic characteristics of a tumor to estimate the probability of achieving breast and axillary pCR. We used a 5-fold cross-validation model in a cohort of patients. We assigned the largest value of the Youden index as the cutoff and the nomogram score was 97 points. In this cutoff, sensitivity was 91.2%, specificity was 67.2%, positive predictive value was 41.9%, negative predictive value was 96.7% and accuracy was 72.2%. The generated data demonstrated that the incidence of a low clinical T stage, negative PR, positive HER2 and clinical radiologic node response were all significant independent predictors of ypT0/ypN0 on an adjusted analysis.
No association in breast and axillary pCRs was found with a clinical tumor response in our study. This may indicate that it is the degree of the initial tumor size, which predicts a known biological response in the axilla and the breast. In our study, a stable disease and a PD seemed to be a meaningless designation for comparison, because there was no case of pCR, but it was difficult to find the meaning in the analysis. Moreover, the cases of CR and PR were more than 50% in the incidence of a non-pCR. Interestingly to note, among 471 axilla pCR patients, 202 patients (42.9%) have breast and axilla pCRs. However, among the 242 breast pCR patients, 202 patients (84.2%) have breast and axilla pCRs. In line with this reasoning, only 40 patients (15.8%) indicative of breast pCR have nonaxilla pCR.
During our study period, it is noted that the trastuzumab was not added to the NAC as a standard treatment in patients with HER2 positive tumors. For the most part, the neoadjuvant trastuzumab therapy was approved in late 2013 in Korea, making it a relatively new treatment option for patients requiring this treatment. Therefore, only 10.0% of the patients in our study received trastuzumab with their NAC. In addition, the patients of the HER2 positive breast cancer had 29.5% (95 of 322) both pCR but these of HER2 negative breast cancer had 16.3% (110 of 673) both pCR. The American College of Surgeons Oncology Group Z1071 trial, which reflects current chemotherapy approaches including the use of trastuzumab for patients with HER2 positive tumors, showed differential nodal responses based on the tumor biology. The overall nodal pCR was 41.1%, but this varied from 21.1% in patients with Hormone receptor (HR) positive/HER2 negative tumors, to 49.4% in patients with a triple-negative breast cancer to 64.7% in patients with HER2 positive disease (P < 0.0001) [12]. Given these points, it was shown that recently a meta-analysis showed that improvement in event-free survival for pCR vs non-pCR was substantial: (HR, 0.37). This association was greater for patients with a hormone receptor-negative disease (HR, 0.29) than for those patients who had a hormone receptor-positive disease (HR, 0.52) [13]. Although the biologic tumor subtype was associated with pCR, we did not subsequently incorporate the biologic tumor subtype into the nomogram. Because the definition of biologic subtype varies somewhat among studies, we chose to include the receptor status (ER, PR, and HER2) of biologic subtype from the nomogram for simplicity purposes. It is important to point out that our study corroborated these findings, with a noted positive HER2 status that was strongly predictive of a tumor and axillary response.
Many studies have evaluated nomograms for predicting the axillary response to NAC in node positive patients with breast cancer [14151617]. A recent study showed that patients with high nuclear grade (odds ratio [OR], 13.4), HER2-positive (OR, 4.7), ER- negative (OR, 3.5), or PR-negative (OR, 4.3) tumors were more likely to achieve axillary pCR [14]. A study of 403 patients with biopsy-confirmed axillary metastases who received NAC, showed that patients who achieved a axillary pCR had received a better 5-year overall survival (93 %) and diseasefree survival (87%) than those who had acquired the residual axillary disease (72%, 60%) [7]. The axillary pCR is affected by offering a better oncological outcome of the patients. However a recent study demonstrated that the pCR defined as no residual invasive cancer in the breast and axillary nodes with presence or absence of in situ cancer (ypT0/is ypN0 or ypT0 ypN0), served to provide a better association with improved outcomes as compared to eradication of an invasive tumor from the breast alone (ypT0/is) [18]. Therefore, the evaluation of both breast and axilla pCRs is more important than the evaluation of only the axilla pCR.
Although this study was performed in a single comprehensive cancer institution located in Korea and the number of patients was relatively small, this study did not investigate clinically suspected node-positive patients, but instead investigated node-positive patients whose diagnoses were confirmed by cytology. Therefore the results from the small number of participating patients are still meaningful, and allow us to confidently draw important results. Additionally this study was not a prospective randomized clinical trial, and for that reason, the distribution of patients might be uneven and can be presumed to have had some effect on the results of the regional control. Our study did not includ the regimens commonly used for neoadjuvant, therapy of docetaxel, carboplatin, trastuzumab, and pertuzumab in HER2 positive breast cancer, since it included patients who would have been in a follow-up of patients monitored up until 2014. Also, we had 238 patients with sentinel lymph node biopsy (SLNB) try and 85 patients with SLN negative. Analyses of several prospective trials show that the accuracy of SLNB can be improved using a dual-tracer technique and the retrieval of 2 or more SLNs, with an ensured removal of the node initially confirmed to contain metastases [3]. Several studies currently are working on ways to ensure removal of the clipped nodes, while using techniques such as a targeted axillary dissection [41920]. We did not perform a targeted axillary dissection in our study. If these better techniques were used, then a more accurate axillary evaluation would be possible in future studies.
In conclusion, the breast and axilla pCRs are associated significantly with a low clinical T stage, negative PR, positive HER2 and a clinical radiologic node response. Our nomogram might help predict breast and axilla pCRs after NAC in patients with initially node-positive breast cancer. Patients with a high probability of achieving both breast and axillary pCRs might be candidates for less invasive surgery.
Figures and Tables
Fig. 1
Nomogram for the prediction of the probability of the pathologic complete response. PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.

Fig. 2
The receiver-operating characteristics (ROC) curve and the calibration plot of the nomogram in the training set. (A) ROC curve with area under the curve = 0.868 (95% confidence interval, 0.845–0.891). (B) Calibration plot of the nomogram.

Fig. 3
The receiver-operating characteristics (ROC) curve of the nomogram in the cross-validation set. (A) ROC curve with area under the curve = 0.853 (95% confidence interval, 0.837–0.869). (B) Calibration plot of the nomogram.
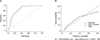
Table 1
Baseline patient and tumor characteristic of 995 patients treated with neoadjuvant chemotherapy

References
1. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15:2483–2493.


2. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.

3. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.


4. Mittendorf EA, Caudle AS, Yang W, Krishnamurthy S, Shaitelman S, Chavez-MacGregor M, et al. Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014; 21:2468–2473.


5. Kim KS, Kim Z, Shim EJ, Kim NH, Jung SY, Kim J, et al. The reality in the follow-up of breast cancer survivors: survey of Korean Breast Cancer Society. Ann Surg Treat Res. 2015; 88:133–139.



6. Shen J, Gilcrease MZ, Babiera GV, Ross MI, Meric-Bernstam F, Feig BW, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007; 109:1255–1263.


7. Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005; 23:9304–9311.


8. Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–1310.


9. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609.


10. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007; 25:3657–3663.


11. Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005; 23:5983–5992.


12. Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014; 260:608–614.


13. Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016; 2:751–760.

14. Vila J, Mittendorf EA, Farante G, Bassett RL, Veronesi P, Galimberti V, et al. Nomograms for predicting axillary response to neoadjuvant chemotherapy in clinically node-positive patients with breast cancer. Ann Surg Oncol. 2016; 23:3501–3509.


15. Kantor O, Sipsy LM, Yao K, James TA. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018; 25:1304–1311.


16. Kim JY, Park HS, Kim S, Ryu J, Park S, Kim SI. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine (Baltimore). 2015; 94:e1720.

17. Schipper RJ, Moossdorff M, Nelemans PJ, Nieuwenhuijzen GA, de Vries B, Strobbe LJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno) therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014; 14:315–322.

18. Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015; 22:1441–1446.


19. Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015; 261:378–382.

20. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016; 34:1072–1078.







 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download