Abstract
Background and Purpose
The initial diagnosis of medullary infarction can be challenging since CT and even MRI results in the very acute phase are often negative.
Methods
A retrospective, observer-blinded study of horizontal conjugate eye deviation was performed in 1) 50 consecutive patients [age 58±15 years (mean±SD), 74% male, National Institutes of Health Stroke Scale 2±1] with acute unilateral lateral medullary infarction as seen in MRI (infarction group), 2) 54 patients with transient brainstem symptoms [transient ischemic attack of brainstem (TIA) group; age 69±16 years, 59% male], and 3) 53 patients (age 59±20 years, 49% male) with diagnoses other than stroke (control group).
Results
Conjugate eye deviation was found in all patients in the infarction group [n=47 (94%) with ipsilesional deviation and n=3 (6%) with contralesional deviation] compared to 41% (n=22) in the brainstem TIA group and 15% (n=8) in the control group (p<0.0001). Within all groups mean deviation and range were similar for both sides (to the right vs. to the left side 26.6°±12.3 vs. 26.1°±12.3 in the infarction group, 10.5°±5.8 vs. 8.4°±6.3 in the brainstem TIA group and 4.5°±3.2 vs. 7.5°±3.2 in the control group). The extent of eye deviation was significantly greater in the infarction group (p<0.05).
Conclusions
All patients with MRI-demonstrated unilateral medullary infarction showed conjugate eye deviation. Therefore, conjugate eye deviation in patients with suspected acute lateral medullary infarction is a helpful sensitive sign for supporting the diagnosis, particularly if the deviation is >20°.
Ischemic brainstem strokes constitute 10% of all ischemic brain strokes with medullary infarction, thus accounting for 7% of all brainstem strokes. Making the initial diagnosis can frequently be challenging due to large variations in the extent of clinical manifestations. CT results are commonly negative in ischemic brainstem stroke.1 Moreover, 30% of patients with clinical diagnoses of brainstem stroke have false-negative diffusion-weighted imaging (DWI) findings, especially within the first 72 hours after symptom onset.2
Horizontal conjugate eye deviation as a tonic displacement of both eyes away from the midline toward one side is a well-known phenomenon in unilateral infra- and supratentorial central lesions. Partial and forced conjugate eye deviations are found in 6% and 27% of the patients, respectively, more frequently in right hemispheric stroke.34 Conjugate eye deviation has also been described in lesions affecting the vestibular system.567 However, the exact role of conjugate eye deviations in brainstem lesions remains less clear, and their usefulness in diagnosis remains to be systematically examined.
The aim of this retrospective observer-blinded study was to determine the frequency and extent of horizontal conjugate eye deviation in the initial imaging in patients with acute unilateral lateral medullary infarction compared to patients with transient brainstem symptoms and patients with diagnoses other than stroke.
We analyzed the clinical and imaging data of consecutive patients treated in our neurological department. Patients and controls were excluded if no appropriate evaluation of the eyes was possible due to technical reasons, which resulted in a dropout rate of about 10% for screened patients and controls. The patient group with MRI-demonstrated infarction in the lateral medulla was compared to a brainstem transient ischemic attack (TIA) group consisting of patients with transient neurological brainstem symptoms and no evidence of infarction in MRI, and to a control group consisting of patients with normal CT/MRI findings and diagnoses other than stroke. The imaging data were analyzed by an experienced neuroradiologist who was blinded to the diagnosis and evaluated an anonymized set of single CT/ MRI slices on which only the eyes were seen. The eye deviation angle was analyzed with GeoGebra software (version 4.2; https://www.geogebra.org) (Fig. 1) and measured in angular degrees between the midline and equator of the lens. The mean value of deviation of both eyes was used for further analysis. We classified the deviations into mild (1–10°), moderate (11–20°), and severe (>20°).
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). One-way ANOVA (for nonparametric values) was performed to compare the mean values of conjugate eye deviation in the three groups. Differences were considered significant if p<0.05. This study was approved by the Local Ethics Committee and was performed in accordance with their ethical standards (project number 18-055).
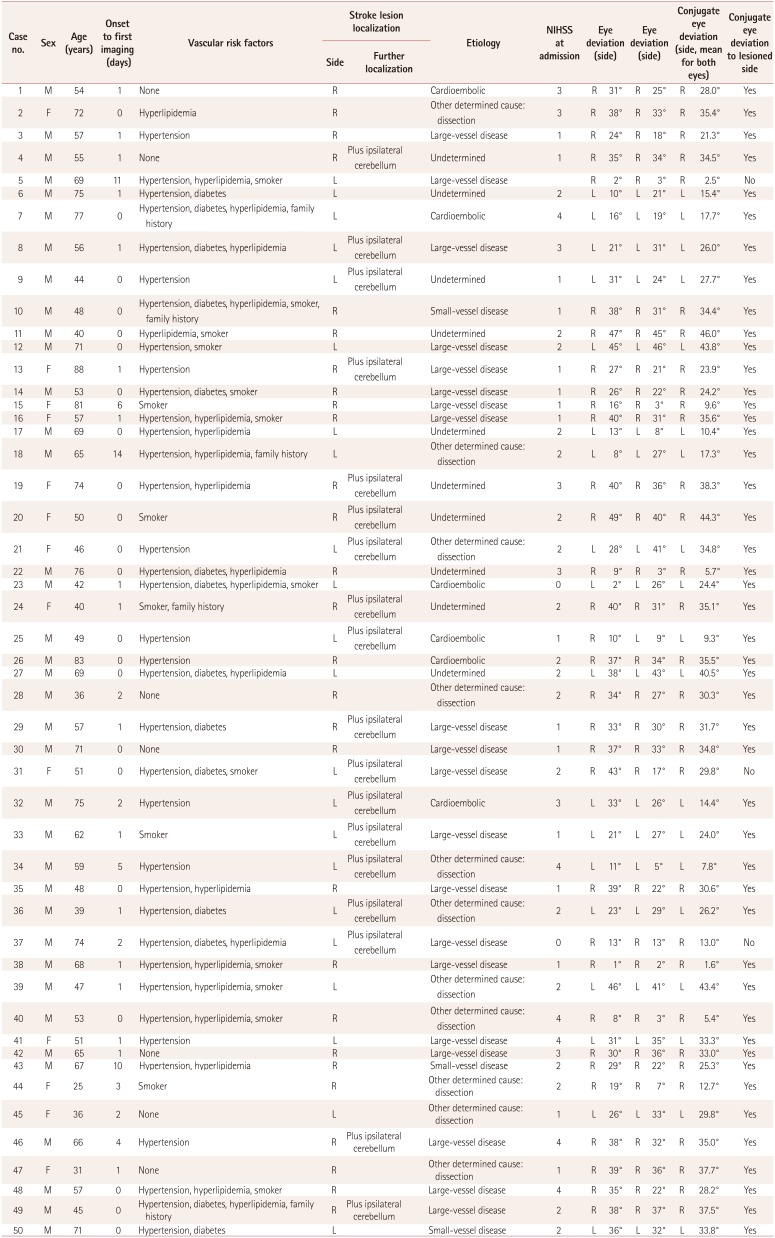
Three groups of patients were examined and compared in terms of conjugate eye deviation as determined by CT or MRI. Fifty patients with MRI-demonstrated unilateral lateral medullary infarction (infarction group) were identified during the study period [age 58±15 years (mean±SD), 74% male, National Institutes of Health Stroke Scale (NIHSS) 2±1; for further details see Table 1]. An initial CT scan was obtained for 43 patients (35 within 1 day after symptom onset, 5 on days 1–5, and 3 on days 5–10), with no infarctions identified; initial MRI was performed in the other 7 patients (5 within 1 day and 2 within 5 days). A purely medullary infarction was found in 31 patients (19 right-sided and 12 left-sided), and 19 patients had an additional ipsilateral cerebellar infarction (8 right-sided and 11 left-sided).
Ipsilateral conjugate eye deviation to the side of the lesion was found in 47 (94%) of those in the infarction group, while the other 3 (6%) patients showed conjugate eye deviation contralateral to the lesion (3°, 4°, and 25°; all left-sided). Mean deviation and extent were similar to the right (26.6°±12.3) and to the left (26.1°±10.6). The deviation was severe in 68% (21 to the right and 13 to the left), while 7 patients had moderate (2 and 5 respectively) and 9 mild deviation (5 and 4 respectively).
The group was compared to 54 patients in the brainstem TIA group (age 69±16 years, 59% male, NIHSS 1.4±0.7). Conjugate eye deviation was present in 41% of those in this group (13 on the right side and 9 on the left side). Mean deviation was 10.5±5.8° to the right and 8.4±6.3° to the left. The deviation was mild in 61% (8 to the right and 4 to the left), while 8 patients had moderate (5 and 3 respectively) and 2 severe deviation (0 and 2 respectively). Other eye deviations observed were single-eye deviation (n=8) and disconjugate deviation (n=23).
The control group consisted of 53 patients (age 59±19 years, 49% male, NIHSS 0.7±1.3): 8 with benign paroxysmal positional vertigo, 6 with acute vestibular syndrome, 6 with epilepsy, 7 with functional disorders, and 26 with other conditions. Conjugate eye deviation was observed in 15% (n=8), while 15% (n=8) had disconjugate eye deviation and 17% (n=9) had single-eye deviation. The conjugate eye deviation was 4.5°±3.2 to the right and 7.5°±3.2 to the left side, and all cases were classified as mild (5 and 3 respectively).
The incidence of conjugate eye deviation was significantly higher in both the infarction and the brainstem TIA groups compared to the control group (p<0.001) (Fig. 2). In addition, the deviation was more severe in the infarction group, whereas the control group showed only mild deviations of ≤10°. Furthermore, eye deviations other than conjugate eye deviation were observed in the brainstem TIA and the control groupss. Therefore, there were significant intergroup differences regarding the incidence of conjugate eye deviations as well as in their severity and extent (all p<0.05).
The main findings of this study, which performed the first standardized workup of eye deviation in such a large group of patients, are described below.
Firstly, all of the infarction patients had conjugate eye deviation, compared to 42% of those in the brainstem TIA group and 15% in the control group.
Secondly, the extent of the eye deviation was significantly higher in the patient group than in the control group. Therefore, looking at the eye position in the initial imaging may give the neurologist and radiologist an indication for a diagnosis of posterior circulation stroke. Furthermore, in patients with suspected acute lateral medullary infarction conjugate eye deviation could be a helpful sign and support the diagnosis, particularly if the deviation is >20°.
The above findings are in line with those of a previous investigation in patients with Wallenberg syndrome using videooculography that found conjugate eye deviation toward the lesioned side in 91% of patients when the eyes were closed.8 This phenomenon can be used to reveal conjugate eye deviation in clinical examination with only a low prevalence of 6% which has been reported in 130 patients with lateral medullary infarction.9 The presumed mechanism behind the phenomenon of conjugate eye deviations is a lesion within the olivary projections to the contralateral vestibulocerebellum.10 Approximately one-third (38%) of our patients had an additional ipsilateral cerebellar infarct. Radiographic conjugate eye deviation was described in 37% of 35 patients with acute cerebellar infarction, mostly contralateral to the lesion, particularly among patients with infarction of the PICA territory including the flocculonodular lobe and/or vermis.11 In another case series of 19 patients with acute vestibular syndrome, radiographic conjugate eye deviation was reportedly present in most of those patients (with 2 patients excluded from the further analysis), of whom 11 had central lesions (10 with stroke and 1 with multiple sclerosis) and 6 had peripheral vestibulopathies (all vestibular neuritis).7 However, although the range of conjugate eye deviation was higher in patients with central lesions, those authors were not able to discriminate between central and peripheral lesions.7 In contrast, in our larger cohort comparing only acute lateral medullary infarction to brainstem TIA patients and control subjects we were able to demonstrate that the occurrence of conjugate eye deviation is helpful in diagnosing acute lateral medullary infarction, particularly if the conjugate eye deviation is >20°.
Thirdly, three of the present patients had contralateral conjugate eye deviation. There has been only one case report on a patient with upper medial medullary infarction and contralateral conjugate eye deviation.12 Those authors attributed the contralateral eye deviation to a synergic mechanism involving the nucleus prepositus hypoglossi, the inferior olivary nucleus, flocculus, and vestibular nucleus.
Fourthly, 42% of the current brainstem TIA patients showed conjugate eye deviation, which could be best explained by transient brainstem dysfunction. Since 30% of patients have false-negative DWI imaging in the very acute stage,2 some of these patients still could have an infarction. The clinical implication of finding conjugate eye deviation in patients with suspected brainstem TIA is that they should be followed up after 48–72 h.
This study has several limitations. Firstly, it has a retrospective design. Secondly, the frequency of eye deviation in other brainstem and cerebellar infarctions as well as in the subgroup of acute vestibular syndrome (and especially peripheral lesions) was not examined. Thirdly, since the patients did not receive any specific instructions, it cannot be ruled out that some of them had their eyes open during the scan or accidentally moved their eyes horizontally, which may have biased the analysis and reduced both the number of patients with eye deviation and the extent of eye deviation. Fourthly, the possibility of clinical overlap between the brainstem TIA and control groups also cannot be ruled out.
In conclusion, this study shows that conjugate eye deviation observed in initial imaging can guide the clinician toward a diagnosis of lateral medullary infarction. This is highly relevant from a clinical point of view, because CT is the most commonly used imaging modality in patients with acute brainstem stroke, and it normally does not allow the diagnosis of an acute brainstem infarction within the first hours; however, the present study has revealed that eye deviation may allow such a diagnosis.
References
1. Bryan RN, Levy LM, Whitlow WD, Killian JM, Preziosi TJ, Rosario JA. Diagnosis of acute cerebral infarction: comparison of CT and MR imaging. AJNR Am J Neuroradiol. 1991; 12:611–620. PMID: 1688347.
2. Sylaja PN, Coutts SB, Krol A, Hill MD, Demchuk AM. VISION Study Group. When to expect negative diffusion-weighted images in stroke and transient ischemic attack. Stroke. 2008; 39:1898–1900. PMID: 18420957.

3. Singer OC, Humpich MC, Laufs H, Lanfermann H, Steinmetz H, Neumann-Haefelin T. Conjugate eye deviation in acute stroke: incidence, hemispheric asymmetry, and lesion pattern. Stroke. 2006; 37:2726–2732. PMID: 17008621.
4. Ringman JM, Saver JL, Woolson RF, Adams HP. Hemispheric asymmetry of gaze deviation and relationship to neglect in acute stroke. Neurology. 2005; 65:1661–1662. PMID: 16301502.

5. Kim JS, Moon SY, Park SH, Yoon BW, Roh JK. Ocular lateropulsion in Wallenberg syndrome. Neurology. 2004; 62:2287. PMID: 15210896.

6. Crevits L, Vander Eecken H. Ocular lateropulsion in Wallenberg's syndrome: a prospective clinical study. Acta Neurol Scand. 1982; 65:219–222. PMID: 7080807.

7. Kattah JC, Pula J, Newman-Toker DE. Ocular lateropulsion as a central oculomotor sign in acute vestibular syndrome is not posturally dependent. Ann N Y Acad Sci. 2011; 1233:249–255. PMID: 21951001.

8. Dieterich M, Brandt T. Wallenberg's syndrome: lateropulsion, cyclorotation, and subjective visual vertical in thirty-six patients. Ann Neurol. 1992; 31:399–408. PMID: 1586141.

9. Kim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003; 126:1864–1872. PMID: 12805095.

10. Solomon D, Galetta SL, Liu GT. Possible mechanisms for horizontal gaze deviation and lateropulsion in the lateral medullary syndrome. J Neuroophthalmol. 1995; 15:26–30. PMID: 7780568.

11. Nishimura K, Ohara T, Nagatsuka K, Minematsu K, Toyoda K. Radiographic conjugate horizontal eye deviation in patients with acute cerebellar infarction. J Neurol Sci. 2015; 355:68–71. PMID: 26026945.

12. Ogawa T, Ueno Y, Kamo H, Miyamoto N, Yamashiro K, Tanaka R, et al. Conjugate eye deviation caused by upper medial medullary infarction: a case report. J Stroke Cerebrovasc Dis. 2018; 27:e221–e223. PMID: 29861128.

Fig. 1
CT and MRI scan of patients. A: CT scan of a middle-aged patient with right dorsolateral medulla infarction, which was later demonstrated in MRI (eye deviation angle α of 47° on the right side and 38° on the left side), with the eyes closed. B: MRI scan of a middle-aged patient with right dorsolateral medulla infarction (eye deviation angle β of 37° on the right side and 35° on the left side), with the eyes closed.
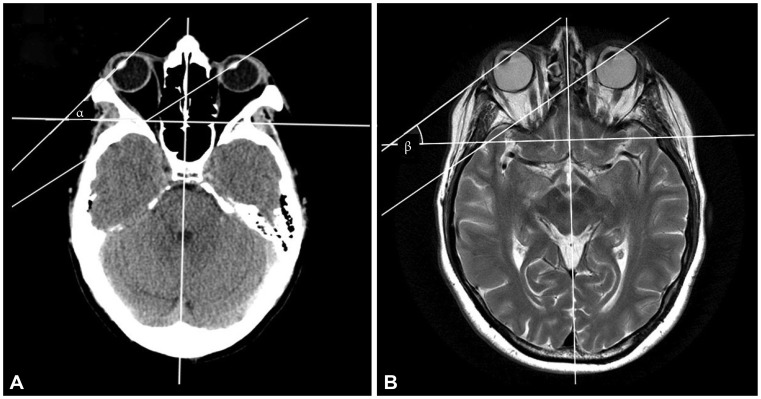
Fig. 2
Overview of study results. A: Results of conjugate eye deviation in the three compared groups (lateral medullary infarction, demonstrated in MRI; brainstem TIA group; and control group). B: Comparison of mean of conjugate eye deviation. *In MRI, purely medullary infarction was found in 31 patients (19 right-sided, 12 left-sided); 19 patients had an additional ipsilateral cerebellar infarction (8 right-sided, 11 left-sided). NIHSS: National Institutes of Health Stroke Scale, TIA: transient ischemic attack of brainstem.
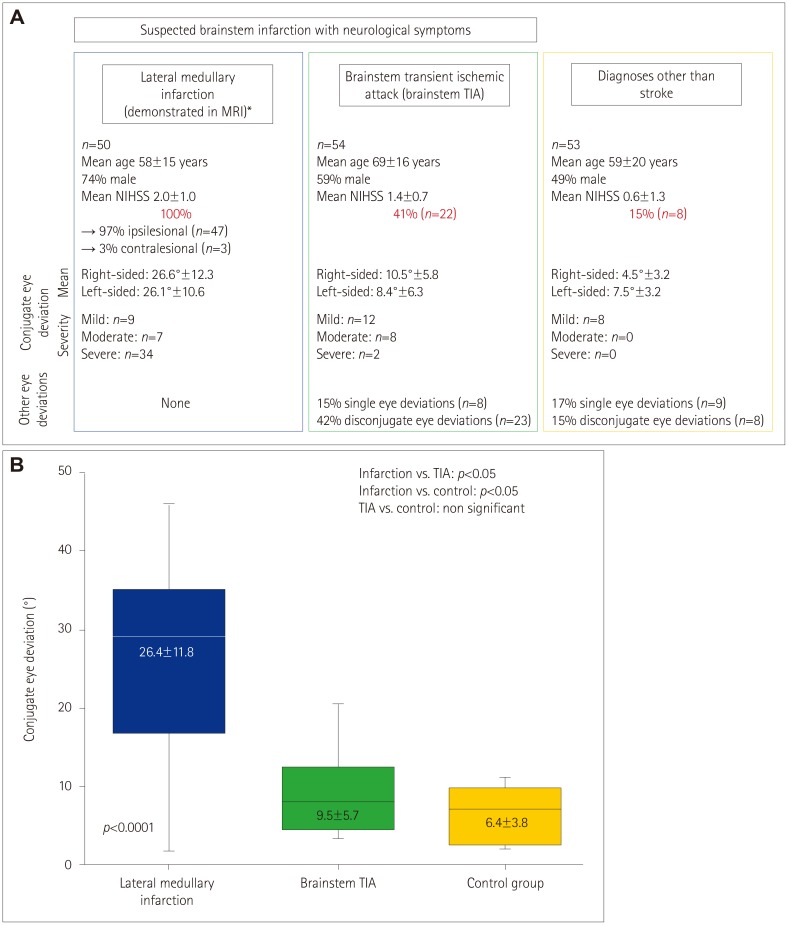
Table 1
Clinical characteristics of the patient group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download