INTRODUCTION
Pertussis, or whooping cough, is a respiratory illness caused by infection with highly contagious
Bordetella pertussis. Infants are at particular risk of infection before completing the primary DTaP (diphtheria toxoid, tetanus toxoid and acellular pertussis) vaccination, and also experience the highest rates of morbidity and mortality compared to the rest of the population.
1 The incidence of pertussis tends to peak every 3–5 years, although several countries have reported an increase in the overall incidence of disease in older children, teenagers and adults, prompting expansions to the available vaccination programs in the past ten years.
234 The tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis booster vaccine (Tdap, Boostrix®; GSK, Rixensart, Belgium) was licensed in 2005 for use in adolescents and adults in the United States (US),
5 and recommendations were expanded to include pregnant women since 2011 to stimulate maternal antibody transfer for the protection of neonates.
267 A similar program was implemented in the United Kingdom (UK) in 2012 following a sharp increase in pertussis cases.
89 Cocooning strategies, involving vaccination of parents and others in close contact with newborns, were also attempted although proved logistically difficult to implement.
2310
The disease burden of pertussis in Korea declined substantially with the implementation of infant DTaP vaccination in 1982, and Tdap registration for adults and adolescents in 2010. As in other countries, however, recent outbreaks occurred in 2012 and 2015, and the incidence is continuing to rise in 2018. The most recent reports from the Korean Centers for Disease Control and Prevention (KCDC) show 230 confirmed cases in 2012, 205 in 2015 and 318 in 2017.
11 Routine passive surveillance during 2001–2012, and ad hoc active sentinel surveillance during the 2012 outbreak, showed infants under one year of age had the highest age-specific incidence of pertussis.
12 A noticeable increase in the proportion of adolescents and adults (> 20 years) with pertussis was observed after 2011.
12 As a result, the KCDC recently updated their immunization guidelines to include Tdap vaccination of pregnant women and of adults and adolescents in close contact with infants, in order to reduce the risk to neonates and young infants.
13
In Korea, Tdap vaccine is indicated for adolescents and adults older than 10 years of age, for the prevention of diphtheria, tetanus, and pertussis. Tdap or Td (without acellular pertussis) is included in the National Immunization Program for adolescents aged 11–12 years.
14 The primary series for infants includes DTaP with polio vaccine at ages 2, 4 and 6 months, with one booster dose of DTaP each at 15–18 months and 4–6 years of age.
15
The aim of this study was to assess the safety of Tdap (Boostrix®; GSK) in pre-adolescents, adolescents, adults (including pregnant women), and older adults (65 years or older), when administered in routine practice in the post-marketing setting. The incidence of adverse events (AEs), including unexpected and serious AEs (SAEs), and factors considered to affect the safety of Tdap vaccination were collected. This study was conducted in compliance with regulatory requirements of the Ministry of Food and Drug Safety (MFDS).
RESULTS
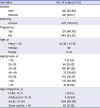
Data from 672 subjects were assessed for safety, while data from 10 subjects who did not fulfill inclusion/exclusion criteria were analyzed separately. There were no pediatric, geriatric or pregnant subjects, or cases with a hepatic or renal disorder among the 10 separately analyzed subjects. All 672 subjects in the safety analysis were Korean. There were 451 women subjects (67.11%) of which 211 (46.78%) were pregnant. The mean age of subjects was 44.38 ± 14.76 years, ranging from 11 to 81 years of age (
Table 1).
Table 1
Demographic data
|
Variables |
No. of subjects (%) |
|
Gender |
|
|
Men |
221 (32.89) |
|
Women |
451 (67.11) |
|
Ethnicity |
|
|
Korean |
672 (100.00) |
|
Pregnancy |
|
|
Yes |
211 (46.78) |
|
No |
240 (53.22) |
|
Age, yr |
|
|
Mean ± SD |
44.38 ± 14.76 |
|
Median |
38 |
|
Range |
11–81 |
|
Age groups, yr |
|
|
< 20 |
7 (1.04) |
|
20–29 |
64 (9.52) |
|
30–39 |
281 (41.82) |
|
40–49 |
73 (10.86) |
|
50–59 |
110 (16.37) |
|
≥ 60 |
137 (20.39) |
|
≥ 75 |
18 (2.68) |
|
Age categories, yr |
|
|
Child: 10–11 |
1 (0.15) |
|
Adolescent: 12–18 |
4 (0.60) |
|
Adult: 19–64 |
604 (89.88) |
|
Older adults: ≥ 65 |
63 (9.38) |

During the entire surveillance period, 90 subjects experienced 124 AEs, resulting in an AE incidence of 13.39%. A majority of AEs (58.87%) were unlikely to be related to vaccination, while 51 events in 35 subjects (5.21%) were assessed by clinicians as related (i.e., 8 certainly, 23 probably, and 20 possibly) to vaccination (i.e., 51 ADRs). These included 40 administration site reactions in 30 subjects, three respiratory events in two subjects possibly related to vaccination (i.e., common cold, coughing, rhinorrhea), one subject each with diarrhea, fever, chills, fatigue, rash, and, arthralgia, and two subjects with myalgia. The most common AEs assessed as not related to vaccination were; 14 constipation events, 14 respiratory system events (e.g., common cold and coughing) and 13 women reproductive disorder events (e.g., premature labor, leukorrhea, vaginal hemorrhage). Six SAEs occurred in six subjects (0.89%). Two subjects had a vaginal hemorrhage, and one subject each had gastroenteritis, premature birth, genital infection, and, fever. All except fever were unexpected SAEs (
Table 2). None of the SAEs were assessed as related to vaccination. (
Supplementary Table 1).
Table 2
Summary of events over the study period (n = 672 total vaccinated cohort)

|
Variables |
No. of AEs |
No. of subjects (%) |
95% CI (for the % of subjects) |
|
AEs total |
124 |
90 (13.39) |
10.91–16.20 |
|
Unexpected AEs |
65 |
54 (8.04) |
6.09–10.35 |
|
SAEs |
6 |
6 (0.89) |
0.33–1.93 |
|
Unexpected SAE |
5 |
5 (0.74) |
0.24–1.73 |
|
ADRs total |
51 |
35 (5.21) |
3.65–7.17 |
|
Unexpected ADR |
10 |
8 (1.19) |
0.52–2.33 |
|
SADR |
0 |
0 (0) |
0.00–0.55 |

Of the 118 non-SAEs, 94.92% were classed as mild, and the remaining six AEs as moderate (i.e., three injection site events, one case each of common cold, pre-eclampsia, and rash). Most AEs (73.73%) did not result in medically-attended visits, however, one subject with enterocolitis went to the Emergency room, and medical personnel were consulted for 30 AEs (25.42%). In most cases, subjects recovered from the AE (120/124 AEs, 96.77%) but recovery status was unknown for 4 AEs (i.e., one case each of gastroesophageal reflux, premature birth, arthralgia, and pyuria).
Overall, 54 subjects (8.04%) had 65 unexpected AEs; five subjects (0.74%) had an unexpected SAE assessed as not related to vaccination, while eight subjects (1.19%) had 10 unexpected non-serious ADRs (
Table 2). The most commonly observed unexpected AEs assessed as not related to vaccination were 24 gastrointestinal system disorders (e.g., constipation in 14 subjects, 2.08%), followed by 13 women reproductive disorder events as described previously and, eight respiratory system disorders (e.g., three cases of common cold, two of dyspnea and two of pleural effusion). Unexpected SAEs included two subjects with vaginal hemorrhage and one each with premature birth, genital infection and gastroenteritis. The most common ADR was seven injection site events in six subjects, followed by one subject with a common cold, one with rhinorrhea and one with chills (
Table 3).
Table 3
Most frequent unexpected AEs reported in ≥ 2 cases (n = 672 total vaccinated cohort)

|
Unexpected AEs |
No. of AEs |
Subjects |
Related to vaccination |
|
No. (%) |
95% CI (for the % of subjects) |
|
Application site disorders |
|
|
|
|
|
Injection site pruritus |
3 |
3 (0.45) |
0.09–1.30 |
2 Probable |
|
1 Possible |
|
Injection site rash |
2 |
2 (0.30) |
0.04–1.07 |
1 Probable |
|
1 Possible |
|
Gastrointestinal disorders |
|
|
|
|
|
Constipation |
14 |
14 (2.08) |
1.14–3.47 |
Unlikely |
|
Dyspepsia |
5 |
5 (0.74) |
0.24–1.73 |
Unlikely |
|
Enterocolitis |
2 |
2 (0.30) |
0.04–1.07 |
Unlikely |
|
Respiratory system disorders |
|
|
|
|
|
Common cold |
4 |
4 (0.60) |
0.16–1.52 |
1 Possible |
|
Dyspnea |
2 |
2 (0.30) |
0.04–1.07 |
Unlikely |
|
Pleural effusion |
2 |
2 (0.30) |
0.04–1.07 |
Unlikely |
|
Women reproductive disorders |
|
|
|
|
|
Premature labor |
4 |
4 (0.60) |
0.16–1.52 |
Unlikely |
|
Leukorrhea |
2 |
2 (0.30) |
0.04–1.07 |
Unlikely |
|
Vaginal hemorrhage |
2 |
2 (0.30) |
0.04–1.07 |
Unlikely |
|
Urinary system disorders |
|
|
|
|
|
Pyuria |
2 |
2 (0.30) |
0.04–1.07 |
Unlikely |

Safety analysis in special populations
In terms of special populations with AEs, the study included five pediatric subjects (< 19 years) (three of them reported an AE: injection site pruritus, injection site pain, and headache), 63 geriatric subjects aged 65 years or older (12 of them reported 16 AEs), 18 geriatric subjects aged 75 years and over (three of them reported five AEs), 211 pregnant subjects (45 of them reported 59 AEs unrelated to vaccination), 13 subjects with renal disorders (two of them reported two mild injection site ADRs), and, 51 subjects with hepatic disorders (10 of them reported 9 injection site ADRs and 4 AEs unrelated to vaccination). The difference in AE incidence was not statistically significant by presence of renal disorder (P = 0.689) or hepatic disorder (P = 0.175).
Safety analysis was conducted in 211 pregnant women (mean age, 33.10 ± 3.32 years; ranging from 25 to 43 years), of which 50.2% had previous pregnancies. The mean gestational week was 38 ± 2 weeks at the time of Tdap vaccination, ranging from 26 to 41 weeks. Nearly half (48.34%) had a medical condition at the time of pregnancy, the most frequent being ‘pregnancy, puerperium, and perinatal conditions’ in 67 subjects (65.69%), followed by ‘endocrine disorders’ in 23 subjects (22.55%) and ‘gastrointestinal disorders’ in 19 subjects (18.63%). Pregnancy outcome of ‘live infant, no apparent congenital anomaly’ was observed in 195 subjects (92.42%) and ‘lost to follow-up’ in 16 subjects (7.58%). The infants' mean birth weight was 2,943 ± 623 g, ranging from 660 to 4,440 g.
A total of 45 pregnant subjects reported 59 AEs, resulting in an AE incidence of 21.33%. None of the reported AEs was an ADR. Most AEs were classed as unexpected (46 AEs reported by 39 subjects). The most frequent unexpected AE (6.64%) was constipation in 14 subjects, followed by dyspepsia and premature labor each in 4 subjects (1.90% each), and dyspnea, pleural effusion, leukorrhea, vaginal hemorrhage, and pyuria, each in 2 subjects (0.95% each). There were six SAEs in six pregnant subjects (2.84%), none were SADRs. Two subjects had a vaginal hemorrhage (0.95%) and 1 subject each (0.47% each) had gastroenteritis, premature birth, genital infection, and fever. Women with a medical condition at the time of pregnancy were significantly more likely to report AEs than those without a medical condition (45 AEs in 32 subjects with conditions versus 14 AEs in 13 subjects without, P = 0.001). Most AEs occurred among subjects with ‘pregnancy, puerperium, and perinatal conditions’, followed by those with ‘gastrointestinal disorders’ and finally ‘endocrine disorders’.
Factors affecting safety
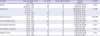
The results indicated differences in the incidence of AEs for six factors including: gender, pregnancy, current medical history, concomitant medication, concomitant vaccination, and age group (
Table 4). Vaccination history was not found to affect safety. In the month prior to vaccination, 32 subjects (4.8%) were administered other vaccines; primarily against typhoid (26 subjects, 81.25%) followed by hepatitis vaccines (12 subjects, 37.50%), measles vaccines (9 subjects, 28.13%), and influenza and papillomavirus vaccines (4 subjects each, 12.5% each).
Table 4
Incidence of AEs by background factors
|
Variables |
Total vaccinated cohort (n = 672) |
No. of AEs |
No. of subjects with AE |
% of AEs (95% CI) |
P values |
|
Gender |
Men (n = 221) |
25 |
18 |
8.14 (4.90–12.57) |
0.005 |
|
Women (n = 451) |
99 |
72 |
15.96 (12.71–19.68) |
|
Age group, yr |
10–11 (n = 1) |
1 |
1 |
100.00 (2.50–100) |
0.009 |
|
12–18 (n = 4) |
2 |
2 |
50.00 (6.76–93.24) |
|
19–64 (n = 604) |
105 |
75 |
12.42 (9.89–15.31) |
|
≥ 65 (n = 63) |
16 |
12 |
19.05 (10.25–30.91) |
|
Geriatric group, yr |
< 75 (n = 654) |
119 |
87 |
13.30 (10.79–16.15) |
NS (0.722) |
|
≥ 75 (n = 18) |
5 |
3 |
16.67 (3.58–41.42) |
|
Current medical history |
Yes (n = 431) |
100 |
70 |
16.24 (12.89–20.07) |
0.004 |
|
No (n = 241) |
24 |
20 |
8.30 (5.14–12.53) |
|
Concomitant medication |
Yes (n = 445) |
103 |
74 |
16.63 (13.29–20.42) |
0.001 |
|
No (n = 227) |
21 |
16 |
7.05 (4.08–11.19) |
|
Concomitant vaccination |
Yes (n = 128) |
5 |
5 |
3.91 (1.28–8.88) |
0.001 |
|
No (n = 544) |
119 |
85 |
15.63 (12.68–18.95) |
|
Pregnant women |
Yes (n = 211) |
59 |
45 |
21.33 (16.00–27.48) |
0.004 |
|
No (n = 240) |
65 |
27 |
11.25 (7.55–15.94) |

The AE incidence by gender was higher among women (15.96%) than men (8.14%) (P = 0.005). The incidence of AEs among pregnant women (21.33%) was higher than among non-pregnant women (11.25%) (P = 0.004).
Overall, 64.14% of subjects in the study had a current medical history; the most common were related to metabolism and nutrition (e.g., diabetes, overweight, osteoporosis, vitamin D deficiency) in 217 subjects, followed by vascular in 151 subjects (e.g., atherosclerosis, hypertension, coronary artery stenosis), gastrointestinal in 123 subjects, endocrine in 106 subjects, musculoskeletal and connective tissue in 93 subjects, psychiatric disorders in 87 subjects, pregnancy, puerperium, and perinatal conditions in 53 subjects, and, reproductive system and breast disorders in 46 subjects. The AE incidence was higher among subjects with a current medical history (16.24%) than in subjects without (8.30%) (P = 0.004).
A total of 445 subjects (66.22%) used concomitant medications, the most common were for the cardiovascular and hematopoietic system in 254 subjects, central nervous system in 228 subjects and gastrointestinal and hepatobiliary system in 226 subjects. The incidence of AEs was higher among subjects using concomitant medication (16.63%) compared with subjects without concomitant medications (7.05%) (P = 0.001).
A total of 128 subjects (19.05%) had one or more concomitant vaccinations, the most common being influenza vaccines in 101 subjects, pneumococcal vaccines in 16 subjects and varicella zoster vaccines in 8 subjects. The incidence of AEs was higher among subjects without concomitant vaccinations (15.63%) than among those with concomitant vaccinations (3.91%) (P = 0.001).
The incidence of AEs was 12.42% among adults aged 19–64 years and 19.05% among older adults (65 years and over). Very few children and adolescents were enrolled in the study. Two AEs were reported in 2/4 adolescent subjects and 1 AE in 1/1 child subject (P = 0.009).
There were 10 subjects excluded from the safety analysis set as the study vaccine was not administered according to the prescription information in Korea (i.e., study vaccine administered into the buttock): four of these subjects experienced five AEs (i.e., injection site pain, common cold, myalgia and two generalized weakness events). Of those, three were ADRs (i.e., injection site pain, generalized weakness and myalgia). No SAE was reported.
DISCUSSION
This six-year post-marketing surveillance in Korea monitoring the safety of Tdap (Boostrix®; GSK) in children, adolescents and adults demonstrated an acceptable safety profile of the vaccine. The overall incidence of any AE was 13.39%, of which unexpected AEs occurred in 8.04% of subjects, and there were six SAEs, none of which were related to vaccination. The incidence of AEs for which the causal relationship to vaccination could not be ruled out was 5.21%, of which the majority were mild administration site reactions.
As this was an observational study of routine clinical practice, many subjects had a current medical condition (64%) or used concomitant medication (66%). Subjects with these factors had a higher incidence of AEs, as did women versus men, pregnant women versus non-pregnant women or older adults versus other adult groups. The incidence of AEs among children and adolescents should be interpreted with caution given the low number of subjects in these age groups (i.e., four adolescents and one child). The difference in AE incidence by gender was consistent with previous diphtheria or tetanus vaccine studies which also reported a higher rate of adverse reactions in women than in men.
18 Given the nature of post-marketing surveillance, and due to possible confounding variables, it was not possible to determine the clinical significance of these factors alone on AE incidence.
19
Safety data were available for special populations such as pregnant women. AEs occurred in 21.33% of pregnant women (constipation was most frequently reported) and were assessed as not related to vaccination. Having a current medical condition during pregnancy was associated with a higher risk of AEs. Six pregnant women reported a SAE including two cases of vaginal hemorrhage and a premature birth, none of which were assessed as related to vaccination. A recent study has found that more women in Korea are having children at an older age, and with increased risks of preterm delivery observed in women aged over 30 years, or low birth weight babies for mothers over 35 years old.
20 The safety of Tdap vaccination in pregnant women has not been previously assessed in Korea, however, data from large US
26 and UK
8 studies have not found an increased risk of AEs following Tdap vaccination during pregnancy. In a randomized double-blind placebo-controlled trial assessing the safety of maternal Tdap vaccination in infants, there were no AEs or negative impact on infant growth and development versus controls (infants of mothers not vaccinated with Tdap during pregnancy).
3 In large retrospective observational studies in the UK and the US covering over 46,000 women, maternal Tdap vaccination was not associated with any increased risk of preterm birth, small for gestational age (SGA) birth or any other AEs related to pregnancy or to any differences in neonatal outcomes such as birth weight, compared with unvaccinated subjects.
368 In fact, one study in the US of over 7,000 pregnant women vaccinated with Tdap found no adverse pregnancy, delivery, or neonatal outcomes associated with vaccination, but a significantly higher average gestational age at delivery, fewer births before 35 weeks of gestation, a shorter duration of neonatal hospitalization, and a lower incidence of SGA neonates.
2 Routine Tdap vaccination has been recommended by the CDC in the US since 2011 during each pregnancy, and it is currently also recommended in other countries (e.g., New Zealand, Australia, the UK, Belgium, Brazil, Mexico, and Argentina).
2122232425
Post-marketing safety data from a retrospective database review in 10–18 year old adolescents (n = 13,427) given Tdap in routine practice in the US found no increased risk of medically-attended neurologic or hematologic events, allergic reactions, chronic illnesses or deaths following Tdap, compared with matched historical controls given Td (tetanus, diphtheria) vaccination.
19
This study was unique in that it assessed the safety of Tdap (Boostrix®; GSK) among Koreans vaccinated in a real-world setting that included pregnant women. This observational study collected safety data from routine clinical practice. Given the design and nature of the post-marketing surveillance, results should be interpreted cautiously, as there was no control group and it was not possible to control for confounding factors.
In this six-year post-marketing surveillance, administration of Tdap (Boostrix®; GSK) to adults and children 10 years or older, including pregnant women, for the prevention of diphtheria, tetanus and pertussis is shown to have a well-tolerated safety profile. In the future, AEs and their causal relationship to Tdap will continue to be monitored through routine spontaneous reporting.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download