This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The perigastric vagus nerve may play an important role in preserving function after gastrectomy, and intraoperative neurophysiologic tests might represent a feasible method of evaluating the vagus nerve. The purpose of this study is to assess the feasibility of neurophysiologic evaluations of the function and viability of perigastric vagus nerve branches during gastrectomy.
Materials and Methods
Thirteen patients (1 open total gastrectomy, 1 laparoscopic total gastrectomy, and 11 laparoscopic distal gastrectomy) were prospectively enrolled. The hepatic and celiac branches of the vagus nerve were exposed, and grabbing type stimulation electrodes were applied as follows: 10–30 mA intensity, 4 trains, 1,000 µs/train, and 5× frequency. Visible myocontractile movement and electrical signals were monitored via needle probes before and after gastrectomy. Gastrointestinal symptoms were evaluated preoperatively and postoperatively at 3 weeks and 3 months, respectively.
Results
Responses were observed after stimulating the celiac branch in 10, 9, 10, and 6 patients in the antrum, pylorus, duodenum, and proximal jejunum, respectively. Ten patients responded to hepatic branch stimulation at the duodenum. After vagus-preserving distal gastrectomy, 2 patients lost responses to the celiac branch at the duodenum and jejunum (1 each), and 1 patient lost response to the hepatic branch at the duodenum. Significant procedure-related complications and meaningful postoperative diarrhea were not observed.
Conclusions
Intraoperative neurophysiologic testing seems to be a feasible methodology for monitoring the perigastric vagus nerves. Innervation of the duodenum via the celiac branch and postoperative preservation of the function of the vagus nerves were confirmed in most patients.
Trial Registration
Clinical Research Information Service Identifier:
KCT0000823
Keywords: Vagus nerve, Gastrectomy, Intraoperative neurophysiological monitoring
INTRODUCTION
The perigastric vagus nerve is frequently sacrificed during radical gastrectomy, and this is known to cause postgastrectomy syndromes [
1]. Efforts have been made to preserve the vagus nerve to maintain the function of the stomach in the treatment of early gastric cancers, especially in pylorus-preserving gastrectomy in Japan and Korea, where early cancer accounts for a large proportion of gastric cancer diagnoses [
234].
The clinically important parts of the perigastric vagus nerve are the hepatic branch and the celiac branch [
56]. The benefits of preserving the hepatic branch during surgery include the prevention of postoperative gallstone formation and reducing delays in gastric function in pylorus-preserving gastrectomy [
378]. Berthoud et al. [
5] evoked motility responses in rats, and found that the hepatic branches were most concentrated in the duodenum while presenting minor projections to the distal antrum of the stomach and intestine. In contrast, the celiac branches are connected to the jejunum, ileum, cecum, and entire colon, and along with other vagal branches, extend to the duodenum [
5]. In humans, the celiac branch is connected to the celiac trunk, and a complex plexus is observed around the left gastric artery and common hepatic artery, which may connect the celiac branch to the duodenum or the hepatic branch [
69].
Preserving functional connections from the celiac branch to the duodenum or hepatic branch may provide benefits for maintaining duodenal motility after gastrectomy. Such benefits have been observed in experiments in dogs by Ando et al. [
10]. In addition, Nunobe et al. [
2] included preservation of the celiac branch in pylorus-preserving gastrectomy procedures and reported that such preservation reduced delayed emptying as compared to historical results. However, another study has reported such procedures are ineffective in preserving the celiac branch [
11].
The celiac branch is also known to be associated with intestinal motility and diarrhea after gastrectomy. However, reports on the ability of celiac preservation to mitigate incidence of diarrhea or weight loss are conflicting [
8121314151617]. These discrepancies could be partly explained by possible unnoticed injuries to the vagus nerve during surgery. However, previous studies have not evaluated the functional distribution or viability of the vagus nerve branches during surgery in humans.
Intraoperative electrophysiologic studies represent a tool for evaluating the function and viability of the nerve and have been applied in various surgeries, such as thyroid or spinal surgeries. In these operations, the viability of the nerve can be immediately identified by obtaining electrical patterns from the corresponding skeletal muscles [
1819]. Previous studies for vagus nerve have stimulated and recorded evoked responses in the vagus nerve at the level of the neck [
202122]. However, the vagus nerve at the level of the abdomen is too short to record direct action potentials or provoke peristalsis in the smooth muscles of the gastrointestinal organs. Therefore, monitoring the viability and/or function of the nerve in the abdomen is challenging [
23]. To date, human studies have not stimulated or monitored the intra-abdominal vagus nerve.
We have succeeded in stimulating the vagus nerve and observing peristalsis in the corresponding intestines in pilot trials. Subsequently, we designed this study to evaluate the feasibility of performing intraoperative evaluations of the function and viability of the hepatic and celiac branches of the vagus nerve during gastrectomy using an electrophysiologic stimulation test method.
MATERIALS AND METHODS
A total of 13 patients were enrolled in this study. The first 2 tests were performed on patients who had undergone open and laparoscopic total gastrectomy to examine the technical feasibility of the operation. The inclusion criteria were revised starting with the third patient to limit the included patients to 20–75 years of age, with clinical stage T1N0M0 gastric cancer. The exclusion criteria included a history of bleeding or the continuous intake of anticoagulation or antiplatelet therapies in the 3 months prior to enrollment, uncontrolled diabetes, parkinsonism or any other neurologic disease, or a history of upper gastrointestinal surgery. This study was initiated after the approval of the Institutional Review Board of Seoul National University Hospital (H-1306-114-500), and informed consent was obtained from all patients. The protocol was registered in the Clinical Research Information Service of Korea (
https://cris.nih.go.kr, No.
KCT0000823).
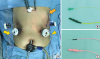
Propofol-based total intravenous anesthesia without muscle relaxants was used for general anesthesia. An upper midline incision was performed in the first case. For the remaining laparoscopic cases, the patients were positioned in a supine position with their head elevated by 15–30 degrees. A pneumoperitoneum was performed through a 12 mm camera port in the umbilicus (
Fig. 1). Two additional ports were inserted in the right and left abdominal walls (sized 12 and 5 mm, respectively) following standard procedures used in laparoscopic gastrectomy. One additional port was added for the liver retractor in selected cases.
Fig. 1
Port sites and introduction of electrodes. (A) Two stimulating electrodes (yellow arrowhead) and 2 recording electrodes (yellow arrow) were introduced in the peritoneal space through 12-mm sized trocars. Two electrodes were placed under the skin in the left upper abdominal wall for grounding and reference (orange arrow). (B) Stimulating electrode. (C) Recording electrode.
The liver was retracted via a laparoscopic liver retractor or by the manual lifting of the liver by an assistant (
Fig. 2,
Supplementary Video 1). The fascia placida was opened, and the subhepatic area was explored to identify and expose the hepatic branches of the vagus nerve. The layer between the right crus and the lesser curvature of the stomach was opened, and the celiac branch of the vagus nerve was dissected via cold endo-scissors and an ultrasonic device (Harmonic scalpel ACE
®; Ethicon Inc., Somerville, NJ, USA). Direct contact between the active blade of the ultrasonic device and the nerve was avoided if possible. Two stimulating probes (APS stimulator; Medtronic, Minneapolis, MN, USA) were inserted via one of the 12 mm trocars and were positioned to grab isolated nerve branches. Two disposable 27-gauge needle electrodes (Medtronic) were placed at the left upper abdominal wall for grounding and reference. Another two 27-gauge needle electrodes (Medtronic) were inserted via another 12 mm trocar and placed at the muscular layer of the intestinal wall for the subsequent detection of peristaltic movement of the intestine [
23].
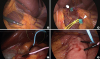
Fig. 2
Placement of electrodes. (A) Hepatic and celiac branches of the vagus nerves were dissected and exposed through the lesser sac. (B) Stimulating electrodes were placed at the hepatic branch (arrow) and the celiac branch (arrowhead). (C) Intramuscular placement of recording electrodes at the first portion of the duodenum. (D) Proximal jejunum.
Intraoperative nerve monitoring systems (Sierra Wave®; Cadwell, Kennewick, WA, USA and Eclipse®, Medtronic) was used to deliver the stimulating current to the nerves and record the signal of the muscular activity of the bowel during reactive movements. A series of 5 square-wave stimuli were delivered, with a duration of 1 ms, an interstimulus interval of 50 ms, and an intensity of 10–30 mA. The myocontractile responses to the stimulation were evaluated by visual inspection, and by the electromyography (EMG) response of the bowel recorded by needle probes at the antrum, pylorus, first portion of the duodenum, jejunum, and 20 cm from the Treitz ligament over 5 consecutive trials of train stimulation at a 1 Hz train repetition rate. The filter for EMG recording was set to 1–3,000 Hz.
In the case of distal gastrectomy, both the hepatic and celiac branches of the vagus nerve were carefully preserved and D1+ lymph node dissection was performed. Careful effort was made to preserve the nerve tissues around the common hepatic artery and the root of the left gastric artery if possible. After dissection, the vagus nerve branches were stimulated and observations were repeated at the duodenum and the jejunum.
The maximal amplitude, latency between the stimulation and the reaction, and the duration of the reaction were analyzed from the recorded graph by an experienced neurologist.
After the operation, standard postoperative care was given to the patients, including sips of water on the 3rd postoperative day, 2 days of a semifluid diet, and a soft-blended diet afterwards. Postoperative complications were checked for 3 months and categorized according to the Clavien-Dindo classification.
Surveys with the Gastrointestinal Symptom Rating Scale with 7 Likert scores (1: no troublesome symptoms to 7: severe troublesome symptoms) and Sigstad's questionnaires were completed preoperatively, on the 3rd postoperative week, and on the 3rd postoperative month to check for the presence and severity of gastrointestinal discomfort, especially diarrhea, loose stool, or dumping syndrome [
242526].
RESULTS
Among the 13 enrolled patients, 11 showed responses to stimulation of the branches of the vagus nerve. The other 2 patients did not show any responses to the stimulation. The myocontractile responses based on the stimulated nerve, anatomic site, and time (preoperative or postoperative) of the 11 patients showing responses are summarized in
Table 1. Most of the responses were identified as visible peristaltic movement as well as electrophysiological signals of myocontractile movement. Certain responses produced myocontractile movement, which could be detected via electrophysiological signals; however, the movements were relatively weak, which increased the difficulty of achieving agreement among the observers. The results (“±”) are presented in
Table 1.
Table 1
Summary of the responses to the stimulation of the celiac branch or hepatic branch of the vagus nerve

|
No. |
Age/sex |
Operation |
Status |
Celiac branch |
Hepatic branch |
|
Antrum |
Pylorus |
Duodenum |
Jejunum |
Antrum |
Pylorus |
Duodenum |
Jejunum |
|
1 |
M/52 |
OTG |
Before |
+ |
+ |
+ |
+ |
− |
|
+ |
+ |
|
2 |
M/59 |
LTG |
Before |
+ |
+ |
+ |
+ |
|
− |
+ |
− |
|
3 |
M/48 |
LDG |
Before |
+ |
+ |
+ |
− |
− |
− |
+ |
− |
|
After |
|
|
− |
|
|
|
+ |
|
|
4 |
M/46 |
LDG |
Before |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
|
After |
|
|
+ |
+ |
|
|
+ |
− |
|
5 |
M/62 |
LDG |
Before |
+ |
+ |
− |
− |
− |
− |
+ |
− |
|
After |
|
|
− |
− |
|
|
+ |
− |
|
6 |
F/65 |
LDG |
Before |
− |
− |
+ |
+ |
− |
− |
+ |
− |
|
After |
|
|
+ |
+ |
|
|
+ |
− |
|
7 |
F/55 |
LDG |
Before |
+ |
+ |
+ |
+ |
− |
+ |
+ |
− |
|
After |
|
|
+ |
− |
|
|
+ |
− |
|
8 |
F/58 |
LDG |
Before |
+ |
− |
+ |
− |
− |
− |
− |
− |
|
After |
|
|
+ |
|
|
|
− |
− |
|
9 |
M/71 |
LDG |
Before |
+ |
+ |
± |
± |
− |
± |
+ |
− |
|
After |
|
|
± |
+ |
|
|
+ |
− |
|
10 |
M/71 |
LDG |
Before |
+ |
+ |
+ |
− |
− |
+ |
+ |
− |
|
After |
|
|
+ |
± |
|
|
− |
− |
|
11 |
F/52 |
LDG |
Before |
+ |
± |
+ |
− |
− |
− |
+ |
− |
|
After |
|
|
+ |
− |
|
|
+ |
− |
|
Positivity |
|
|
Before |
10/11 |
9/11 |
10/11 |
6/11 |
1/11 |
4/11 |
10/11 |
1/11 |
|
After |
|
|
7/9 |
4/7 |
|
|
7/9 |
0/8 |
|
Negative conversion |
|
|
|
|
|
1/8 |
1/4 |
|
|
1/8 |
|
After the celiac branch was stimulated, 9 or more patients showed peristalsis in the lower part of the stomach passing to the duodenum (
Supplementary Video 1,
Table 1). In 1 patient, peristalsis was not evident in the stomach, but contractile movement was observed in the duodenum and jejunum (case 6). The response of the proximal jejunum was evaluated at approximately 20 cm from the Treitz ligament, and 6 patients showed a response. The representative waves of the electrical signal are shown in
Fig. 3, and the peak latency and amplitude are summarized in
Table 2.
Fig. 3
Electrophysiologic measurement of the myocontractile response. (A) Measurement of the latency and the peak amplitude. A representative wave of response to the stimulation of the (B) hepatic branch and (C) celiac branch is presented.

Table 2
Peak latency and amplitude of the responses

|
Location |
Before gastrectomy |
After gastrectomy |
|
No. |
Peak latency (s) |
Amplitude (µV) |
No. |
Peak latency (s) |
Amplitude (µV) |
|
Mean±SD |
Mean±SD |
Mean±SD |
Mean±SD |
|
Celiac branch |
|
|
|
|
|
|
|
Antrum |
10 |
1.93±0.68 |
2,356±1,979 |
|
|
|
|
Pylorus |
9 |
1.99±1.14 |
1,831±771 |
|
|
|
|
Duodenum |
10 |
2.06±1.04 |
2,067±1,957 |
7 |
2.31±0.90 |
829±442 |
|
Jejunum20 |
6 |
2.38±1.29 |
2,150±1,525 |
4 |
2.37±0.89 |
1,368±1,347 |
|
Hepatic branch |
|
|
|
|
|
|
|
Antrum |
1 |
1.45±NA |
2,800± NA |
|
|
|
|
Pylorus |
4 |
1.76±0.90 |
967±472 |
|
|
|
|
Duodenum |
10 |
1.96±1.06 |
2,089±1,288 |
7 |
2.41±1.98 |
2,109±1,771 |
When the hepatic branch was stimulated, gastric peristalsis was only observed in 1 patient (case 4) in the antrum and contractile movement near the duodenum was observed in 10 patients.
For the 9 patients who underwent distal gastrectomy, stimulation and observations were repeated after the operation. Seven out of the 9 patients showed responses in the duodenum following stimulation of the celiac branch, which implied that the response in the duodenum is caused not only by peristalsis from the stomach but also by signal transference through a separate route. Whether this route occurs along the nerve plexus around the common hepatic artery or inversely originates from the superior mesenteric artery (as indicated by the question mark in
Fig. 4) is not clear.
Fig. 4
Predicted pathway of the vagus nerve. Large arrows indicate the region of stimulation. The linkage from the celiac branch to the pylorus (*) and the jejunum (†), which were observed preoperatively, was disconnected postoperatively in cases 3 and 7, respectively.
*Possible route of connection between celiac branch and duodenum.
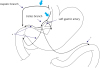
The myocontractile responses following the stimulation of the celiac branch disappeared after gastrectomy in 2 patients. Patient 3 showed a response in the duodenum after stimulation of the celiac branch but did not show a response after gastrectomy. Another patient (case 7) showed negative conversion in the jejunum but preserved responses in the duodenum. This finding suggests that an injury occurred during surgery in the pathway of the celiac branch to the jejunum, which is separate from the route to the duodenum, thus indicating negative conversion did indeed occur via intraoperative injury. After stimulation of the hepatic branch, patient 10 responded in the duodenum preoperatively but lost the response after surgery.
No remarkable events occurred during the procedures, and the exposure of the vagus nerve branches. The application of the electrodes, the stimulation of the nerves, and the observation of the responses all proceeded without incident. During the hospital stays, 1 patient suffered grade II pneumonia, categorized according to the Clavien-Dindo classification. No other patients suffered any adverse complications. The mean duration of the postoperative hospital stay was 7.4±1.5 days (min 5 to max 11).
Surveys administered preoperatively and at 3 weeks and 3 months postoperatively showed no diarrhea or loose stool of grade 4 or higher (moderate or frequent discomfort) (
Fig. 5). In addition, no patients in the postoperative period presented Sigstad scores of more than 6, which are suggestive of dumping syndrome.
Fig. 5
Survey of gastrointestinal symptoms and Sigstad score taken Pre-op., POP 3w, and POP 3 m.
Pre-op. = preoperatively; POP 3 w = postoperatively in the 3rd week; POP 3 m = postoperatively in the 3rd month.

DISCUSSION
This study is the first to evaluate the response of the intestinal tract to the artificial stimulation of perigastric vagus nerve branches.
In this study, the connection between the celiac branches of the vagus nerve and the duodenum was confirmed by the preservation of the response of the duodenum under stimulation of the celiac branch after gastrectomy. Although the celiac branch has been suggested to coordinate the duodenum via the nerve complex around the common hepatic artery, this coordination had not been previously demonstrated in humans. Moreover, the hepatic branch is considered to play a major role in the neural coordination of the duodenum.
In the present study, the duodenum reacted to the stimulation of the celiac branch before surgery as well as after distal gastrectomy, during which the duodenum is disconnected from the nerve of Latarjet at the celiac branch. This finding indicates that connections remained from the celiac branch to the duodenum. Berthoud et al. [
5] reported that the neural distribution of the celiac branch to the duodenum is in the retrospective direction via the superior mesenteric artery. In the present study, one case (case 7) presented a loss of jejunal response but preservation of the duodenal response. This result indicated damage to the celiac branch pathway, which is selective to the jejunum but not the duodenum.
This finding may represent a putative basis for the benefit of preserving the celiac branch during pylorus-preserving gastrectomy to reduce gastric stasis [
2]. However, the benefit was not proven in retrospective analysis, which was likely a manifestation of delayed emptying after pylorus-preserving gastrectomy being primarily associated with a denervated antrum and the function of the pylorus, rather than the emptying function of the duodenum [
16].
Because of simplified illustrations in surgical textbooks or atlases, some surgeons misunderstand the anatomic structure of the posterior vagus nerve. The celiac branch is not completely isolated from the posterior vagus nerve from the level of the esophagus, although surgeons tend to believe that the celiac nerve, which is found near the right crus, is already an isolated branch. However, 1–4 gastric branches extend from the posterior trunk, and the level of the branching part is located close to the left gastric artery [
6], which explains the peristaltic response from the body and the antrum of the stomach when we stimulate the nerve branch around the level of the right crus. More precisely, we have stimulated the posterior nerve trunk, although it is described as the celiac branch for ease of understanding in terms of the role of each branch. Ruckley also reported that the posterior vagus may have one or more hepatic branches that reach the hepatic plexus either independently or by joining the anterior hepatic branches. Therefore, the posterior vagal nerve is not only limited to the stomach and the small bowel but also plays a role in controlling the entire upper gastrointestinal tract, including the duodenum and the hepatic area. These anatomical structures of the posterior vagus nerve might have been ignored due to the limited clinical implications during radical gastrectomy; however, these structures may be meaningful if full-thickness partial resection with sentinel node navigation can be applied in the near future.
In this study, we evaluated the connection and viability of the nerve branch by visual inspection and muscular electrical signals measured via needle probes. If technology is available, measuring the action potentials of the nerve itself may provide more accurate results [
20]; however, the length of the nerve branches may be too short for corresponding nerves connected to each part of the intestine to be traced. This method is more difficult to implement than other intraoperative nerve monitoring methods using skeletal muscles, such as monitoring of the recurrent laryngeal nerve, because the vagal efferent nerve uses thin and slow fibers to evoke peristalsis in the smooth muscles of the intestine [
2728]. The method of stimulating the nerve refers to the methodology of gastric electrical stimulation for gastroparesis [
29]. Individual variability was observed in the reactivity to the stimulation methods, and intestinal movement was evoked by stimulation with the following properties: intensity of 10–30 mA; 4 trains for each stimulation; 1,000 µs for each train, and frequency of 5 times (1 Hz). These settings were tested in several pilot cases before this study.
Two patients did not respond at all to a variable range of stimulation. Unfortunately, among the many potential causes of this lack of response, including damage to the very thin wire of the stimulating probes during insertion through the laparoscopic trocar, inadequate contact between the probe and the nerve, partial damage of the nerve during isolation, anatomic variation, or simply individual variation in responses, the causal factor could not be identified. In most responses, visual inspection can identify peristalsis evoked by the stimulation. In certain cases, peristalsis could not be observed by visual inspection even when electrical signals were detected.
Although the proposed method was found to be feasible, several difficulties in its application were observed. First, concerns were raised about the effect of the muscle relaxant on the synapse of the vagus nerve system. We used propofol-based total intravenous anesthesia to minimize the effects of the drug on the neural stimulation, which can increase the expense of the surgery and introduce difficulties resulting from patients that are not fully relaxed. However, cases in which the surgical procedure was significantly interrupted due to the absence of a muscle relaxant were not observed in this series.
Another critical concern is the invasiveness of the procedure. The nerve should be dissected prior to stimulation, which introduces the possibility of damaging the nerve during dissection and/or stimulation by electricity. Although significant postgastrectomy symptoms or diarrhea were not observed, leaving the nerves in place without too much manipulation is preferred to best preserve their structure. Although concerns may be raised about piercing the bowel wall with needle probes, this procedure generally does not result in perforation, and intraabdominal abscesses or leakages were not observed in this series. Due to a lack of simultaneous measuring systems of multiple areas, we had to measure the electrical signal with multiple stimulations for each area, which increased the procedure time.
Finally, many reports have published discordant results about the benefits of vagus nerve preservation. Given the uncertain benefits of vagus nerve preservation and other factors, enrollment in this study was limited to a very small number of volunteers who were dedicated to scientific development. Nevertheless, we believe that a greater understanding and the intraoperative monitoring of perigastric vagus nerves could be helpful for tailored or functional surgery in the near future. Therefore, the next step in our investigation will involve the development of less invasive methods, such as the trans-luminal stimulation of the vagus nerve, thinner or surface-type detection probes, etc.
In conclusion, intraoperative evaluation of the function and viability of the hepatic and celiac branches via direct stimulation was feasible. This method identified connections between the celiac branch and duodenum and showed the viability of both branches in certain patients after vagus-preservation gastrectomy. However, less invasive and convenient methods are still needed for the general application of intraoperative monitoring in vagus-preservation gastric surgery.










 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download