Abstract
Background/Aims
Surgical resection or ablation is recommended for the treatment of early hepatocellular carcinoma (HCC), whereas transarterial chemoembolization (TACE) is frequently used in early HCC ineligible for curative resection. We evaluated the clinical effects and safety of radiofrequency ablation (RFA) shortly after TACE in patients with Barcelona clinic liver cancer (BCLC) stage A HCC.
Methods
Sixty-seven BCLC stage A HCC patients who failed to achieve complete response to TACE as either a first line treatment and who subsequently received RFA at the Konkuk University Medical Center from January 2005 to December 2017 were included. Evaluation indices included treatment response, overall survival rate, recurrence-free survival, prognostic factors, and procedure-related complications.
Results
Median follow-up was 46.9 months. Fifty-four (80.6%) patients were of Child-Pugh class A, and 13 (19.4%) were of class B. Modified UICC stages were I in 10 (14.9%), II in 46 (68.7%), and III in 11 (16.4%) patients. In the 67 study subjects, cumulative recurrence-free survival rates were 86.8%, 55.9% and 29.7% at 1, 3, and 5 years, respectively, and overall survival rates were 100%, 93.4%, and 83.5% at 1, 3, and 5 years, respectively. Tumor size significantly predicted recurrence. No treatment-related death occurred.
Several treatment options have been recommended for patients with hepatocellular carcinoma (HCC). These treatment options can be categorized as curative or palliative, and the curative options available are radiofrequency ablation (RFA), liver transplantation, and tumor resection. Best treatment options are determined based on stage of presentation, and in general, hepatic resection and transplantation are the first-line treatment options for early stage HCC (Barcelona clinic liver cancer [BCLC] stage A). However, surgery is often not possible due to reduced liver function when cirrhosis is present, and liver transplantation is limited by the lack of suitable donors. RFA is commonly considered a curative treatment in patients with cirrhosis and a tumor size of <3 cm and is safe and less invasive than hepatic resection and produces results similar to surgical resection when tumor sizes are <2 cm.1
Transarterial chemoembolization (TACE) is commonly used to treat unresectable HCC, because tumors are highly angiogenic in nature. TACE provides survival benefit in patients with intermediate HCC, though it is usually considered a palliative modality, because it does not cause complete tumor necrosis and tumor recurrence is common, and because repeat TACE can damage liver function and adversely affect survival.2
It has been reported treatments adopted in Korea for early stage HCC deviate from BCLC recommendations as they show bias toward the use of TACE to treat BCLC stage A HCC.3 TACE and RFA have definite benefits in HCC as they are less invasive and have high application values, but are limited when used as separate therapies. However, combined TACE and RFA overcomes these limitations. Several previous studies have reported TACE plus RFA provides better overall survival than RFA or TACE monotherapies,45678 but clear evidence showing combined treatment is a better option than TACE alone is lacking. Accordingly, the effect of TACE plus RFA on outcome in early stage HCC remains controversial. Therefore, in the present study, we studied the clinical outcomes and safety of TACE plus RFA and sought to identify factors predicting the efficacy of combination therapy in patients with BCLC stage A HCC that underwent TACE as a first-line therapy.
This retrospective study was conducted at the Konkuk University Medical Center (Seoul) on HCC patients consecutively treated from September 2005 to May 2017 that met the study entry criteria. These criteria were as follows; 1) BCLC stage A HCC, 2) TACE as the first treatment for HCC (no previous surgery or regional therapy), tumor location, residual liver function after curative resection, and patient refusal to undergo surgery or RFA, 3) RFA shortly after TACE due to incomplete uptake or no uptake, 4) no extrahepatic metastasis, 5) no imaging evidence of tumor invasion into the major portal or hepatic vein branches, and 6) presence of cirrhosis of Child-Pugh class A or B. Patients with an uncontrollable malignancy other than HCC or with cirrhosis of Child-Pugh class C were excluded.
One hundred eighty-eight patients received TACE as a first-line therapy for HCC during the study period. One hundred twenty-one of the 188 were excluded because of complete lipiodol uptake, an unsatisfactory response assessment after TACE, non-receipt of RFA after TACE, or because of BCLC 0 or B HCC. Consequently, 67 patients constituted the study cohort.
TACE and RFA were performed on an inpatient basis by an interventional radiologist. TACE was performed as follows.9 The hepatic artery was catheterized after celiac and superior mesenteric arteriography using a 5-French catheter (Cook Medical, Bloomington, IN, USA), and the tumor feeding artery was then selectively cannulated using a 3-French microcatheter (Microferret; Cook Medical) and embolized by infusing an emulsion of iodized oil (Lipiodol; Guerbet-Aulnay, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea) while sparing most of the hepatic parenchymal arterial supply. This infusion was continued until arterial flow stasis had been achieved and/or iodized oil was visualized in portal branches. Additional embolization was also performed using gelatin sponge particles (1–2 mm in diameter, Gelfoam; Upjohn, Kalamazoo, MI, USA). After embolization, the extent of vascular occlusion and the presence of residual tumor staining were assessed by angiography. One day after TACE, HCC lipiodol uptake was evaluated by CT. When lipiodol uptake deemed unsatisfactory, all visible tumors were subjected to RFA within 24 hours of CT scan assessments.
RFA was performed percutaneously by two expert radiologists with more than five years' experience of RFA for hepatic tumors, using a 17-gauge straight electrode with a 2 or 3 cm exposed tip length (Cool-tip; Valleylab, Boulder, CO, USA) connected to an RF generator (Cool-Tip RF generator; Radionics, Mansfield, MA, USA). Single or multiple overlapping ablations were performed based on lesion size/geometry and imaging feedback to achieve at an ablative margin of 0.5–1.0 cm around target tumors. Time taken and frequency of RF application depended on numbers and sizes of HCC nodules. After RFA procedures, tract ablation was performed during electrode retraction to prevent bleeding and tract seeding. CT was performed immediately after RFA to assess technical success, which was defined as the presence of a non-enhancing area around index tumors of sufficient ablative margin on portal phase CT images.
The efficacy of TACE was evaluated by dynamic CT one day after the final procedure. Response to TACE was classified as partial or no uptake. Overall survival rates and recurrence-free survival rates after RFA were evaluated. After TACE plus RFA, blood chemistry, AFP level, and abdominal CT, ultrasound, or magnetic resonance images were evaluated at 1 month, 3 months, and 6 months on an outpatient basis. In addition, blood test and abdominal imaging findings were evaluated on a six-monthly basis in accord with National Comprehensive Cancer Network guidelines.10
Overall survival was defined as time from commencement of cancer treatment and recurrence free survival as time from the cessation of first-line treatment until the appearance of any sign or symptom of cancer recurrence. Overall survival and recurrence free survival were evaluated using time elapsed after TACE plus RFA. This study was approved by the Institutional Review Board of Konkuk University Medical Center (KUH 1010725).
Cumulative overall survival and recurrence-free survival rates were estimated using the Kaplan-Meier method. Continuous variables are presented as mean±SDs or as medians and ranges. Univariate and multivariate Cox proportional hazard models were used to identify predictive factors (gender, age <65 yr, HBV, recurrence, UICC stage I, TACE and RFA time between TACE and RFA, number of tumor multiplicity [1 or ≥2], tumor size <20 mm, AFP <20 ng/mL, and Child-Pugh classification A) of overall survival and HCC recurrence after TACE plus RFA. P-values of <0.05 were considered significant, and the analysis was conducted using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA).
Baseline characteristics of the 67 patients are summarized in Table 1. Forty-eight (72%) were men and overall median age was 63.7 years. The etiology of underlying liver disease was HBV infection in 68.7%. Fifty-four (80.6%) patients were of Child-Pugh class A and 13 (19.4%) were of class B. Median serum AFP was 7.32 ng/mL and was <20 ng/mL in 48 (71.6%) patients. Tumor size was <2 cm in 10 (15%) patients and ≥2 cm in 57 (85%). Vascular invasion was present in four (6.0%) patients and bile duct invasion in one patient. Most patients (68.7%) were of stage II according to the modified UICC staging system. All patients received TACE before RFA. The reasons for choosing TACE for early stage HCC were as follows; tumor not visualized by ultrasonography (eight patients), a difficult tumor location as regards the technical approach or multiple lesions (28 patients), ascites or poor liver function (13 patients), and patient refusal of surgery or RFA as a first-line treatment (18 patients). After TACE, partial uptake was observed in 63 and no uptake in 4. The mean time between TACE and RFA was two days. CT findings revealed three patients did not achieve complete ablation, and in these three patients, complete ablation was achieved by secondary RFA.
Median follow-up duration after TACE plus RFA was 46.9 months (range, 1–99 months), during which nine patients died; two of HCC and seven of liver cirrhosis progression. Cumulative overall survival rates after TACE plus RFA at 1, 3, and 5 years were 100%, 93.4%, and 83.5%, respectively (Fig. 1). HCC recurrence after TACE plus RFA occurred in 35 patients (52.2%); intrahepatic local recurrence in 6 (17.1%), recurrence in another segment in 28 (80%), and distant metastasis in one patient (2.9%). Twenty-two patients (63%) underwent TACE as a secondary treatment (Table 2). Cumulative recurrence-free survival rates at 1, 3, and 5 years after RFA were 86.8%, 55.9%, and 29.7% (Fig. 2).
Of the 35 recurrences, the recurrence site was adjacent to the treated site in six patients (17.1%) and in another liver segment in 29 (82.9%).
Cumulative 1, 3, and 5-year survival rates in Child-Pugh class A were higher than in Child-Pugh class B (p=0.021) (Fig. 3), and the cumulative survival rate of those without recurrence was greater than that of those with recurrence (p=0.040) (Fig. 4). No mortality occurred among patients without tumor recurrence during follow-up.
Table 3 summarizes the results of univariate and multivariate analyses for predictors of overall survival. Both univariate and multivariate analysis showed that Child-Pugh class A favorably predicted overall survival (hazard ratio [HR], 0.68; p=0.021).
Univariate and multivariate analysis results for predictors of recurrence free survival are summarized in Table 4. Univariate analysis showed UICC stage I, a single tumor, a small tumor size (<20 mm), and Child-Pugh class A favorably predicted disease free survival, and multivariate analysis showed that of these variables tumor size alone significantly predicted recurrence UICC stage I (HR, 0.39; p=0.221), single tumor (HR, 0.41; p=0.135), small tumor size (<20 mm) (HR, 0.40; p=0.020), Child-Pugh class A (HR, 0.39; p=0.088).
This study demonstrates that combined treatment with TACE and RFA provides a safe, effective option for patients with early stage HCC. According to the BCLC staging system, the recommended curative options for early stage HCC (BCLC stage A) are RFA, liver transplantation, and surgical resection, and TACE is recommended for intermediate HCC (BCLC stage B). However, TACE is commonly used to treat early stage HCC (BCLC stage A) in Korea. In the present study, early stage HCC patients were treated with TACE because of medical conditions, such as diminished liver function (Indocyanine Green test R 15), another medical comorbidity, tumor location, or refusal to undergo invasive management.
TACE is somewhat problematic in early stage HCC because complete necrosis is difficult to achieve due to incomplete embolization and the induction of angiogenesis by residual tumor tissues. Well-differentiated HCC with a tumor diameter of less than 3 cm is often predominantly supplied by portal venous blood, whereas advanced HCC is predominantly supplied by arterial blood. Combined TACE plus RFA is being increasingly used for the complementary treatment of HCC, particularly when tumors are large size. This combined technique substantially overcomes the limitations of TACE and RFA monotherapies. The occlusion of arterial flow by TACE probably reduces heat-sink effects during RFA and thus increase ablation volumes,11 and because of its regional nature, TACE can be used to target undetected satellite lesions beyond RFA-induced necrotic zones.12 The therapeutic effects of TACE plus RFA have been described in several reports, for example, Lencioni et al.13 reported successful ablation of HCC tumors ranging in size from 3.5–8.5 cm in 51 (82%) of 62 HCC patients.
Recent studies have suggested TACE plus RFA might be effective in resectable HCC and transplant candidates.14 Early HCC (BCLC stage A) includes patients with a single HCC or with up to three nodules of <3 cm. Currently, when liver function is preserved without vascular or lymphatic invasion, hepatic resection is considered as the standard therapy for early HCC. However, many patients do not satisfy BCLC criteria for hepatic resection because HCC usually occurs in a background of liver cirrhosis. RFA has been recommended as a first-line therapy for single tumors of ≤5 cm,15 but the risk of incomplete tumor necrosis increase with tumor size. It has been hypothesized that the use of TACE prior to RFA might reduce or eliminate heat losses mediated by tissue perfusion, and thus, enlarge regions of RFA-induced coagulation necrosis and improve treatment efficacy for larger tumors as compared with RFA alone.16
In the present study, we evaluated the clinical efficacy and safety of combination therapy for early HCC by assessing long-term outcomes. Cumulative overall survival rates after TACE plus RFA at 1, 3, and 5 years were 100%, 93.4%, and 83.5%, respectively, which compare well with overall survival rates reported in previous studies on TACE plus RFA. Xie et al.17 reported overall survival rates at 1, 2, 3, 4, and 5 years of 97.5%, 89.4%, 84.2%, 80.4%, and 78.7%, respectively, in 487 patients with solitary HCC, and Song et al.18 reported survival rates at 1, 3, and 5 years of 98%, 95%, and 90% in patients with early HCC.
Several studies have demonstrated TACE plus RFA is comparable to RFA alone or resection in terms of improving the survival of HCC patients.1920 In the present study, prognoses were similar to those previously reported. Recurrence of HCC after TACE plus RFA occurred in 35 patients (52.2%) during follow-up, and cumulative recurrence-free survival rates at 1, 3, and 5 years were 86.8%, 55.9%, and 29.7%, respectively. Kagawa et al.21 reported recurrence-free survival rates at 1, 3, and 5 years of 64.5%, 40.1%, and 18.0% after TACE plus RFA and of 75.6%, 41.1%, and 36.4%, respectively, after resection (p=0.010). They suspected that this difference was due to differences in procedurally-associated rates of local tumor progression, which was observed in nine (14.5%) patients in the TACE plus RFA group but was not observed in the resection group. Peng et al.4 reported 1, 3, and 4-year recurrence-free survivals for TACE plus RFA and RFA alone of 79.4%, 60.6%, and 54.8% and 66.7%, 44.2%, and 38.9%, respectively, and found recurrence-free survival was significantly better in the TACE plus RFA group (HR, 0.575; p=0.009).
We believe the high rates of tumor free survival observed in the present study are probably due to the recruitment of largely early stage HCC patients. In most cases, recurrences were intrahepatic and occurred in other liver segments. Furthermore, recurrence was found to be associated with prognosis, which cautions careful follow-up after treatment and the early detection of recurrence are critical for improving patient outcomes.
Several studies have shown Child-Pugh class A, Cancer of the liver Italian program (CLIP) score, albumin, prothrombin time, platelet, serum AFP, and tumor recurrent status are associated with overall survival. In particular, several studies have concluded tumor size, serum AFP, and number of RFA sessions are positively related to tumor recurrence.1722232425 However, in the present study, serum AFP did not significantly impact overall survival or recurrence free survival, though Child-Pugh class A was found to be a significant independent factor of overall survival and tumor size to be a significant independent predictor of tumor recurrence. Child-Pugh class, CLIP score and albumin, prothrombin time, and platelet level are measures of liver function, which suggests liver functional status most influences mortality. Furthermore, our analysis showed tumor size was significantly associated with tumor recurrence after TACE plus RFA.
In the present study, no significant major procedure-associated complication was encountered. In a previous report, it was concluded that as compared with surgical resection, TACE plus RFA is minimally invasive and safe,26 and several authors have reported significantly lower risks of major complications for TACE plus RFA than surgical resection.192728 However, Kim et al.29 reported severe complications and aminotransferase elevation were more common after TACE plus RFA than after TACE. As demonstrated by our results, time between TACE and RFA is an important safety factor and presents a balance between the preservation of liver function on the one hand and enhancing the synergistic effect of combination therapy on the other. No obvious consensus has been reached regarding the optimal timing of RFA after TACE. In a previous study, we demonstrated that an inter-procedural time of only 2 days or less is sufficient to allow recovery of liver functional reserve in patients with cirrhosis,9 and in the present study, mean time between TACE and RFA was 2 days. No major complication occurred and all minor complications were controlled without permanent damage. Thus, TACE plus RFA appears to be relatively safe. On the other hand, the immediate application of RFA after TACE has the advantages of maximizing synergistic effects, reducing hospital stay, and improving patient compliance.
This study is inherently limited by its retrospective nature and the small number of patients included. Furthermore, the study was conducted without a control, and thus, the results obtained did not enable us to conclude that TACE plus RFA is superior to RFA or TACE monotherapy in early stage HCC. It should be added, several treatment modalities can be used to treat early stage HCC based on considerations of liver function, health status, tumor location, and unwillingness to undergo surgery, but that TACE plus RFA is not commonly used to treat patients with BCLC stage A HCC. Summarizing, the present study shows the clinical efficacy of TACE plus RFA is satisfactory in patients with early stage HCC ineligible for curative treatment, and that RFA immediately after TACE is safe and effective in patients that do not achieve complete uptake after TACE.
Figures and Tables
 | Fig. 1Cumulative overall survival after TACE plus RFA. Cumulative 1-, 3-, and 5-year overall survival rates were 96.7%, 82.9%, and 78.8% at 1, 3, and 5 years, respectively. TACE, transarterial chemoembolization; RFA, radiofrequency ablation; mo, months. |
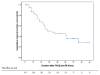 | Fig. 2Cumulative recurrent free survival after TACE plus RFA. Cumulative 1-, 3-, and 5-year recurrence free survival rates were 86.8%, 55.9%, and 29.7% at 1, 3, and 5 years, respectively. TACE, transarterial chemoembolization; RFA, radiofrequency ablation; mo, months. |
 | Fig. 3Cumulative overall survival and Child-Pugh class. Cumulative 1-, 3-, and 5-year survival rates were significantly greater for Child-Pugh class A than class B patients (p=0.021). TACE, transarterial chemoembolization; RFA, radiofrequency ablation; mo, months. |
 | Fig. 4Cumulative overall survival according to HCC recurrence. Cumulative survival rates were significantly higher in patients that did not experience recurrence (p=0.040). TACE, transarterial chemoembolization; RFA, radiofrequency ablation; mo, months. |
References
1. Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008; 47:82–89.


2. Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: barcelona clinic liver cancer staging system. World J Gastroenterol. 2015; 21:10327–10335.



3. Kim SE, Lee HC, Kim KM, et al. Applicability of the BCLC staging system to patients with hepatocellular carcinoma in Korea: analysis at a single center with a liver transplant center. Korean J Hepatol. 2011; 17:113–119.



4. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013; 31:426–432.


5. Georgiades CS, Hong K, Geschwind JF. Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J. 2008; 14:117–122.


6. Lee HJ, Kim JW, Hur YH, et al. Combined therapy of transcatheter arterial chemoembolization and radiofrequency ablation versus surgical resection for single 2-3 cm hepatocellular carcinoma: a propensity-score matching analysis. J Vasc Interv Radiol. 2017; 28:1240–1247.e3.

7. Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011; 53:136–147.


8. Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010; 116:5452–5460.

9. Choe WH, Kim YJ, Park HS, Park SW, Kim JH, Kwon SY. Short-term interval combined chemoembolization and radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2014; 20:12588–12594.



10. Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the national cancer institute clinical trials planning meeting. J Clin Oncol. 2010; 28:3994–4005.

11. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009; 252:905–913.


12. Schwartz M, Weintraub J. Combined transarterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma. Nat Clin Pract Oncol. 2008; 5:630–631.


13. Lencioni R, Cioni D, Donati F, Bartolozzi C. Combination of interventional therapies in hepatocellular carcinoma. Hepatogastroenterology. 2001; 48:8–14.

14. Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008; 8:2547–2557.


15. N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009; 50:1475–1483.


16. Zhu AX, Salem R. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma: one step forward? J Clin Oncol. 2013; 31:406–408.


17. Xie H, Wang H, An W, et al. The efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization for primary hepatocellular carcinoma in a cohort of 487 patients. PLoS One. 2014; 9:e89081.

18. Song MJ, Bae SH, Lee JS, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016; 31:242–252.



19. Pan T, Mu LW, Wu C, et al. Comparison of combined transcatheter arterial chemoembolization and CT-guided radiofrequency ablation with surgical resection in patients with hepatocellular carcinoma within the up-to-seven criteria: a multicenter case-matched study. J Cancer. 2017; 8:3506–3513.



20. Takuma Y, Takabatake H, Morimoto Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013; 269:927–937.

21. Kagawa T, Koizumi J, Kojima S, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010; 116:3638–3644.

22. Sohn W, Choi MS, Cho JY, et al. Role of radiofrequency ablation in patients with hepatocellular carcinoma who undergo prior transarterial chemoembolization: long-term outcomes and predictive factors. Gut Liver. 2014; 8:543–551.



23. Chang NK, Shin SS, Kim JW, et al. Effect of ultrasound-guided radiofrequency ablation in incompletely treated hepatocellular carcinoma after transcatheter arterial chemoembolization. Korean J Radiol. 2012; 13:Suppl 1. S104–S111.

24. Kim JH, Yim HJ, Lee KG, et al. Recurrence rates and factors for recurrence after radiofrequency ablation combined with transarterial chemoembolization for hepatocellular carcinoma: a retrospective cohort study. Hepatol Int. 2012; 6:505–510.

25. Tang C, Shen J, Feng W, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization for unresectable hepatocellular carcinoma: a retrospective study. Medicine (Baltimore). 2016; 95:e3754.
26. Yamakado K, Nakatsuka A, Takaki H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008; 247:260–266.


27. Kim JW, Shin SS, Kim JK, et al. Radiofrequency ablation combined with transcatheter arterial chemoembolization for the treatment of single hepatocellular carcinoma of 2 to 5 cm in diameter: comparison with surgical resection. Korean J Radiol. 2013; 14:626–635.







 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download