TO THE EDITOR: The thymus plays a vital role in cell-mediated immunity by producing functional T cells and inducing self-tolerance. As such, benign or malignant tumors originating from the thymus can lead to loss of self-tolerance and development of autoimmunity [1]. The most common autoimmune disease associated with thymic tumor is myasthenia gravis, followed by pure red cell aplasia (PRCA) [2]. There are reports of other types of immune cytopenias including immune thrombocytopenia [3] and acquired amegakaryocytic thrombocytopenia (AAMT) [4], but because of their rare incidence, the treatment is not well defined. Since thrombocytopenia can cause adverse consequences, patients with low platelet count require prompt clinical attention. Here, we report the case of a patient with invasive thymoma and associated immune thrombocytopenia successfully treated with eltrombopag, a thrombopoietin receptor agonist.
A 69-year old male patient with no significant previous medical history presented to the emergency department with dyspnea. He had been experiencing intermittent edema in the upper extremities for the preceding 3 months but did not seek any medical attention for the symptoms. The chest X-ray taken upon arrival showed cardiomegaly with bilateral pleural effusion. The patient was admitted for further evaluation and echocardiography and chest computed tomography (CT) were undertaken. The images revealed heterogeneous soft tissue enhancing mass in the mediastinum infiltrating the transverse sinus and right para-tracheal area, associated with severe narrowing of superior vena cava, bilateral pleural effusion, and a significant amount of pericardial effusion causing cardiac tamponade (Fig. 1A). There were no other organ involvements. His complete blood count showed marked thrombocytopenia (8×109/L) but normal hemoglobin level (12.2 g/dL) with no neutropenia (white blood cell, 6.3×109/L). After platelet transfusion, pericardiocentesis was performed and dyspnea was relieved. Since the cytology performed with pericardial effusion did not any yield any pathological diagnosis, endobronchial ultrasound biopsy was performed and the World Health Organization (WHO) type B1 thymoma [5] was diagnosed. As the mass was encasing the heart and major vessels, neither operation nor radiation could be the therapeutic options, and thus, the patient was offered chemotherapy with cyclophosphamide, doxorubicin, and cisplatin (CAP) for the treatment. Since there were no other autoimmune diseases and the bone marrow examination obtained prior to chemotherapy showed no metastatic involvement (Fig. 2A), thymoma was thought to be the primary cause of thrombocytopenia.
After the first cycle of chemotherapy, his platelet count recovered to around 50×109/L. The edema subsided and the patient was subjectively feeling better. Consequently, the second cycle of CAP was administered. On the 10th day of follow-up, his platelet count was 58×109/L without other cytopenias. A repeat CT scan was undertaken to evaluate the tumor response, and it showed a reduced size of the mediastinal mass (Fig. 1B).
When the patient visited the clinic for the third cycle of CAP, his platelet count, however, was again low (2×109/L) and he complained of hematochezia. He was immediately admitted for further evaluation. When no recovery in the platelet count was seen after a week of supportive therapy, a second bone marrow examination was performed (Fig. 2B). The bone marrow aspirate at this point showed an adequate number of erythropoietic cells, granulopoietic cells, and megakaryocytes without dysplasia. The bone marrow biopsy showed normocellular marrow areas (30%) and some acellular areas (<10%). Based on these findings, chemotherapy-induced marrow suppression super-imposed on thymoma-associated immune cytopenia was assumed. Prednisolone was started at a dose to treat immune thrombocytopenia (1 mg/kg). After delaying the chemotherapy for 2 more weeks, his platelet count was found to be 69×109/L and he underwent the third cycle of CAP with a 30% reduced dose. After 3 weeks of steroid treatment, he developed steroid-induced myopathy and diabetes mellitus. The steroid was tapered off and cyclosporine plus danazol was initiated. The platelet count level remained somewhat stable ranging from 30–50×109/L, but due to the unpredictable hemogram picture and indolent nature of his tumor, chemotherapy was put on hold indefinitely.
After a month of danazol plus cyclosporine treatment, the patient developed disabling intention tremor preventing him from feeding or dressing by himself. As replacement therapy, azathioprine was administered but his platelet count dropped below 7×109/L, and he was again admitted with massive hematochezia. Despite the low WBC and platelet counts, the reticulocyte count was normal. Intravenous immunoglobulin (IVIG) was initiated for the management of immune thrombocytopenia. IVIG showed immediate effects, and the platelet count increased to 76×109/L.
The hemogram picture and the tumor remained stable for 2 months (Fig. 1C). The patient, however, again presented to the emergency department with hematochezia and severe thrombocytopenia (platelet count, 2×109/L), which were refractory to IVIG treatment. He underwent a bone marrow examination for the third time, and at this point, the marrow showed normal cellularity (30%) with slightly increased megakaryocyte (Fig. 2C). With an affirmative diagnosis of immune thrombocytopenia, eltrombopag was administered at a dose of 25 milligrams (mg) as per recommendation and then increased to 50 mg after 2 weeks of suboptimal response. With a dose of 50 mg, the platelet level was stabilized within 2 weeks and was normalized in another 6 weeks. He is currently on eltrombopag 50 mg and the platelet count is normal and no significant adverse effects of the drug were observed (Fig. 3).
The association of thymic tumors with autoimmune disorders is well-established. It has been reported that up to 30% of patients with thymoma develop autoimmunity during the course of their disease [6]. Cases of autoimmune hematologic phenomena have also been described, most well-known being PRCA [7]. Although in a lesser frequency other hematologic manifestations, such as thrombocytopenia has also been documented. Several mechanisms can cause thrombocytopenia in thymoma, such as 1) secondary to aplastic anemia [8]; 2) immune thrombocytopenia [39]; and 3) acquired amegakaryocytic thrombocytopenia [4]. As immune thrombocytopenia seems to be a rare occurrence in this context [2], there is a lack of a standard treatment for this potentially life-threatening condition.
In previously reported cases of thymoma-associated immune thrombocytopenia, all patients were apt for thymectomy [239] and thrombocytopenia was improved after surgery. Unfortunately, in this case, the mediastinal mass was encasing the large vessels (Fig. 1A), thus the patient was deemed inoperable, and hence, systemic chemotherapy had to be administered. Initially, the patient responded well to chemotherapy, and experienced substantial improvement in the subjective symptoms as the size of the mediastinal mass was reduced. Regardless of the tumor response, the patient, however, developed severe thrombocytopenia in the absence of anemia or neutropenia. Based on the follow-up bone marrow examination, the patient was given immunomodulators (steroids, cyclosporine, azathioprine, and IVIG) usually used in thymoma-associated autoimmune disorders. These agents, however, either cause significant side effects which can compromise the quality of life of the patients or result in less-than-satisfactory outcomes. After all other treatment measures failed, and eltrombopag, a thrombopoietin receptor agonist, was initiated. The rationale of this decision was based on the immediately preceding bone marrow findings which shared typical characteristic features of idiopathic thrombocytopenic purpura (ITP), and the proven efficacy of eltrombopag in chronic ITP [1011]. The patient was administered eltrombopag 50 mg per day and the platelet count recovered without any further complications.
This is, to the best of our knowledge, the first documented use of eltrombopag in a patient with thymoma-associated immune thrombocytopenia, who was refractory to all other therapeutic measures. Our study also highlighted that flares of autoimmune phenomena do not necessarily correlate with the primary tumor response. Although rare, immune-mediated thrombocytopenia can occur in association with thymic tumors. Vigilant workups including bone marrow examination help in making the correct diagnosis leading to appropriate management and good prognosis. Eltrombopag can be useful even in immune thrombocytopenias caused by other underlying diseases in the background of other autoimmune disorders.
Figures and Tables
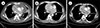 | Fig. 1Chest computed tomography (A) at diagnosis, (B) after three cycles of CAP, and (C) six months after completion of chemotherapy. |
 | Fig. 2(A) Picture of bone marrow at diagnosis. The section shows normocellular marrow without malignant tumor cells (H&E stain, ×400). (B) Second bone marrow examination was performed before the third cycle of chemotherapy. The section (H&E stain, ×400) showed nearly normal distribution of nucleated cells without infiltrative tumor cells. (C) Final bone marrow examination was performed before administration of eltrombopag. The section (H&E stain, ×400) shows normocellular marrow with slightly increased megakaryocytes (right side). |
Acknowledgments
We would like to thank the patient for providing consent to publish this case report.
References
1. Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood. 2000; 96:3872–3879.


2. Rivoisy C, Besse B, Girard N, et al. Thymic epithelial tumor-associated cytopenia: a 10-year observational study in France. J Thorac Oncol. 2016; 11:391–399.


3. Qin J, Liu L. Thymoma with idiopathic thrombocytopenic purpura: report of a case. J Thorac Cardiovasc Surg. 2005; 129:453.


4. Onuki T, Kiyoki Y, Ueda S, Yamaoka M, Shimizu S, Inagaki M. Invasive thymoma with pure red cell aplasia and amegakaryocytic thrombocytopenia. Hematol Rep. 2016; 8:6680.

5. Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg. 2004; 77:1183–1188.


6. Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol. 2011; 8:199–202.



7. Thompson CA, Steensma DP. Pure red cell aplasia associated with thymoma: clinical insights from a 50-year single-institution experience. Br J Haematol. 2006; 135:405–407.


8. Maslovsky I, Gefel D, Uriev L, Ben Dor D, Lugassy G. Malignant thymoma complicated by amegakaryocytic thrombocytopenic purpura. Eur J Intern Med. 2005; 16:523–524.


9. Kobayashi H, Kitano K, Ishida F, et al. Aplastic anemia and idiopathic thrombocytopenic purpura with antibody to platelet glycoprotein IIb/IIIa following resection of malignant thymoma. Acta Haematol. 1993; 90:42–45.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download