Abstract
Background
Sickle cell anemia (SCA) is a hereditary chronic hemolytic anemia with several clinical consequences. Intravascular sickling of red blood cells leads to multi-organ dysfunction. Moreover, several biochemical abnormalities have been associated with SCA. Gum arabic (GA) is an edible dried gummy exudate obtained from Acacia Senegal tree. GA showed antioxidant and cytoprotective activities and demonstrated protection against hepatic, renal, and cardiac toxicities in experimental rats. We hypothesized that regular intake of GA improves renal and liver functions in patients with SCA.
Methods
Forty-seven patients (5–42 yr) carrying hemoglobin SS were recruited. The patients received 30 g/day GA for 12 weeks. Blood samples were collected before administering GA and then after 4, 8, and 12 weeks. Liver enzymes, total protein, albumin, electrolytes, urea, creatinine, and uric acid were determined in the serum. The study was approved by the Al Neelain University Institutional Review Board and Research Ethics Committee Ministry of Health. The trial was registered at ClinicalTrials.gov (identifier: NCT02467257).
Results
GA significantly decreased direct bilirubin level [statistical significance (P-value)=0.04]. It also significantly decreased serum alanine transaminase level after 4 weeks, which was sustained till the 8th week. GA, however, had no effect on serum aspartate transaminase level. In terms of renal function, GA decreased serum urea level but the effect was not sustained after the first month.
Sickle cell disease (SCD) is one of the most common genetic causes of morbidity and mortality in the world [1]. SCD consists of a group of disorders characterized by the presence of sickle hemoglobin. Hemolysis, vaso-occlusive crisis and ischemia-reperfusion injury are the characteristic features of SCD. All these factors lead to cumulative damage of the cerebral, pulmonary, splenic, and renal vasculature, and increase the risk of cerebrovascular accidents, pulmonary hypertension, splenic infarction, and renal injury [2]. Sickle cell anemia (SCA) patients have a high level of oxidative stress markers and low antioxidant capacity [3]. In addition, free radicals may contribute to organ dysfunction and failure.
Several biochemical abnormalities are associated with SCD [4]. Mild elevation in liver enzymes, which is unrelated to age or gender is observed in SCD in the steady state [5]. Several studies have shown that serum bilirubin (total and direct) [6] and liver enzyme levels were significantly higher in homozygous SCD (HbSS) patients than in individuals with normal hemoglobin (HbAA) [4789]. Hepatic dysfunction in SCD is due to multiple factors, such as intra-hepatic sinusoidal sickling, bilirubin gallstones, transfusion-related hepatitis infections, and excess iron deposition [4]. Elevation of the different liver enzymes correlates with different disease process; hemolysis raises plasma aspartate transaminase (AST) while plasma alanine transaminase (ALT) levels more accurately reflect hepatocyte injury [5]. In SCD, AST is released by intravascular hemolysis [4]. Researchers have found an association between the liver size and elevation of the liver enzymes, except for alkaline phosphatase (ALP) in SCD patients [5]. A higher level of ALP may be due to an associated vaso-occlusive crisis involving bones rather than a primary liver pathology [45]. ALP level indicates the severity of bone damage and is a useful marker of the progress of bone pain in SCA [4]. AST:ALT ratio may play a role of a hemolytic marker because it has an inverse association with hemoglobin level [4]. The rise in bilirubin is expected in SCD patients as there is a higher than normal rate of breakdown of red blood cells (RBCs) [6]. Liver microinfarcts due to RBCs sickling could exacerbate the rise in serum bilirubin in these patients [6].
Elevated serum creatinine may be associated with renal insufficiency in SCA patients [4]. Elevated serum creatinine indicates that the disease has reached an advanced stage and is progressing towards renal failure [4]. Patients with SCA or sickle cell trait may suffer from several types of renal dysfunction [4]. In the steady state, serum creatinine [10] and urea tend to be lower in HbSS children than in HbAA ones [411]. HbSS children have higher glomerular filtration rate (GFR) compared to the normal individuals [1011]. Hyperfiltration is present in sickle cell children [1112], which may persist till adulthood in some patients [12]. Glomerular hyperfiltration, regardless of the etiology, may eventually lead to glomerular sclerosis, proteinuria, and progressive renal failure [1013].
In terms of serum electrolytes, a study has found increased serum potassium and decreased serum sodium in SCD patients than in the normal individuals [4]. Abnormality in serum potassium level is seen more commonly in SCA patient with renal insufficiency [4]. Researchers reported that dehydration is one of the main causes of sodium movement inside the sickle cell resulting in hyponatremia [14]. During dehydration and de-oxygenation, excessive potassium is lost from the cells to the extracellular fluids. Dehydration and cation depletion could be the possible cause of sodium loss from the extracellular fluid into the intracellular fluid resulting in hyponatremia and hyperkalemia [14].
Hyperuricemia is a common feature of HbSS disease [15]. Many studies have shown an increased serum level of uric acid (UA) in SCD than in normal individuals [4111516]. The clinical significance of hyperuricemia is not clear, as gout is rare in SCD [151617]. This may be because the SCD patients usually do not live long enough to develop clinical symptoms of gout [17]. It was suggested that an excessive level of serum UA results from an increased marrow activity and turnover of nucleic acids secondary to hemolysis [4]. Since all patients with HbSS disease in the steady state have evidence of increased erythropoietic activity, increased synthesis of UA might be expected [41516]. The major determinant of hyperuricemia in SCA is, however, a reduced renal urate clearance [1518]. Higher serum UA level is found in patients with proteinuria, which may indicate associated tubular damage [1518].
Gum arabic (GA) is defined by the Food and Agriculture Organization-World Health Organization Joint Expert Committee for Food Additives (JECFA) as ‘a dried exudate obtained from the stems of Acacia Senegal tree or closely related species of Acacia (family Leguminosae)’. GA has a beneficial role to modify the physiological system in humans. It is claimed to have many physiological and therapeutic effects which were investigated in several studies [1920]. Previous works have shown that dietary supplementation with GA increases both fetal hemoglobin level and antioxidant capacity of patients with SCA [321]. SCA patients are at risk of multi-organ failure, particularly renal insufficiency and hepatic dysfunction with advancement in age. Therefore, it will be of great benefit to find a drug that provides protection to the vital organs in SCA. The aim of this study was to investigate the possible ameliorative effects of GA on several parameters of renal and liver function in SCA patients.
The study was approved by the Al Neelain University Institutional Review Board and Research Ethics Committee, Khartoum State Ministry of Health. The trial was registered at ClinicalTrials.gov on June 3, 2015 (identifier: NCT02467257).
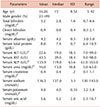
The patients' characteristics and background data are documented earlier [21]. The main outcome of interest was the level of fetal hemoglobin after 12 weeks [21]. The secondary outcomes were an improvement in clinical and laboratory parameters. The daily dose of GA was 30 grams (g), which was administered in one sachet dissolved in 200 milliliters (mL) of water and was consumed in the early morning for 12 weeks. The renal and liver function tests were performed at the baseline and then monthly up to the end of the study.
Three milliliters of blood was withdrawn in lithium heparin container. The blood was allowed to clot at room temperature and the serum samples were transported to the Chemistry Department in the Central Laboratory Military Hospital. Cobras C311 (Roche, Germany) automated chemistry analyzer was used to determine total protein, albumin, ALT, AST, ALP, amylase, creatinine, uric acid, total bilirubin, and direct bilirubin level in serum. The machine used the principles of absorption photometry to determine the absorbance in the blood which was used to calculate the concentration in the solution [22]. Ion-selective electrode was used to estimate serum sodium and potassium levels [22].
The continuous variables data are expressed as range, mean, median, and standard deviation after testing for normality by Kolmogorov-Smirnov test and Shapiro-Wilk test. Repeated measures analysis of variance followed by least significant difference (LSD) multiple comparison tests were used for the normally distributed data. Non-parametric tests were used for the data that were not normally distributed. All analyses were performed using SPSS software package version 21 and a statistical significance (P-value) of <0.05 was considered as statistically significant.
Oral ingestion of GA showed no effect on total bilirubin, although there was a significant decrease in direct bilirubin level (P=0.04) (Fig. 1A, B). GA did not affect serum total protein and albumin levels (Fig. 1C, D). Although GA decreased serum ALT level significantly, the effect was temporary (Fig. 2A). GA had no effects on serum AST or ALP levels (Fig. 2B, C). Although GA decreased serum urea level significantly, the effect was temporary (Fig. 3A). Serum creatinine and electrolyte levels showed no response to oral GA (Fig. 3B, 4A and 4B). GA did not affect serum uric acid level either (Fig. 5).
SCA is syndrome rather than a disease as it affects almost every organ manifested by altered renal and liver functions as compared to the normal individuals (HbAA). Gum arabic, which is a dietary fiber, was found to be protective against hepatic and renal toxicities in experimental rats [1923].
In this study, GA significantly decreased serum direct bilirubin level (Fig. 1A). Dietary fibers are believed to either bind or sequester bile acids, inhibit their active reabsorption in the ileum, and facilitate their excretion in the feces [24]. This may explain why GA decreased direct bilirubin level without any effect on total bilirubin. As a result of hemolysis, the major bilirubin which is elevated in SCA is unconjugated bilirubin [6]. GA did not alter the level of liver enzymes (Fig. 2). GA, however, temporarily suppressed the serum ALT level (Fig. 2), an indicator of hepatic injury [4] (Fig. 2A). These results are consistent with a previous study conducted in healthy volunteers where GA was found to significantly decrease serum ALT level after three weeks [25]. GA protected the liver against chemical-induced hepatotoxicity and significantly decreased serum ALT and AST levels [23] which was attributed to its reactive oxygen species scavenging activity [23]. SCA patients in our study had a normal level of albumin and total protein, and GA had no effect on these parameters (Fig. 1C, 1D). Previous studies have shown that SCD patients have higher total protein and albumin level as compared to the normal individuals [4].
The nephroprotective effect of GA is one of its earliest recognized and scientifically proven beneficial effect. GA was found to increase creatinine clearance significantly and alter electrolyte excretion suggesting favorable actions in renal insufficiency [26]. In our study, GA significantly decreased the serum urea level but the effect was not sustained after the first month probably because most of the patients had a mean urea level between 8–30 mg/dL, which is within the normal reference range. Nevertheless, the serum urea levels in two patients with high serum urea level (38 and 63 mg/dL) were decreased significantly within the first month to 12 and 20 mg/dL, respectively. Reduction in serum urea level could be due to an increased urea nitrogen excretion in the stool [272829]. GA did not affect serum creatinine level (Fig. 3B). SCA patients have lower serum creatinine level as compared to the normal individuals because of higher GFR and increased creatinine clearance [10]. GA had no effects on the serum electrolyte levels (Fig. 4A, B). In this study, the patients had almost normal serum electrolyte levels (Table 1), whereas other studies have shown the presence of hyperkalemia and mild hyponatremia in SCD patients as compared to the normal individuals [4]. GA was found to decrease urinary sodium excretion with no effect on serum sodium level in an animal model [30].
SCA patients may have hyperuricemia because of increased marrow activity and nucleic acid turnover [4]. In this study, the mean serum uric acid level was 5.8 mg/dL (range, 2.3–10.7 mg/dL). GA showed no effect on serum uric acid level (Fig. 5). A study conducted in Sudan have shown that GA decreased UA level in patients with renal insufficiency [24]. This contradictory finding could be explained by the different etiology of hyperuricemia in SCD, which could be attributed to excess hemolysis rather than renal diseases.
Because of the increased average lifespan of SCA patients, the frequency of chronic complications including renal failure, gallstones, and hepatic involvements have increased. Biochemical abnormalities play a significant role in the pathophysiology of SCA which can be targeted for the proper management of SCD [4]. In this study, daily consumption of 30 g of GA decreased serum direct bilirubin level with temporary effect on serum ALT level. There was, however, no effect on total bilirubin and other liver enzyme levels. On the other hand, GA temporarily altered the renal function. Most of the current medications in SCA focus on fetal hemoglobin as the therapeutic target to decrease the incidence of vaso-occlusive crisis and intravascular hemolysis and thereby reduce the disease severity and organ damage. None of the current medications have, however, shown direct protective effects on different organs in SCA. The exact mechanism(s) by which GA acts in SCA is not clear and is most probably multi-factorial with the most important one being antioxidant activities.
The main limitation of this study was that this was a single arm study. A study with multiple arms with a longer duration will be appropriate to explain the hepatoprotective and nephroprotective effects of GA in SCA. In conclusion, GA, which contains soluble dietary fibers having prebiotic properties, might have protective effects against hepatic and renal damage in patients with SCD. This was evidenced by a reduction in serum bilirubin level and temporary reduction in serum urea and ALT levels.
Figures and Tables
 | Fig. 1Effects of GA on liver function (a)significant difference from baseline). (A) Effects of GA on direct bilirubin level (P=0.008). (B) Effects of GA on total bilirubin level (P=0.590). (C) Effects of GA on serum albumin level (P=0.186). (D) Effects of GA on total proteins level (P=0.155). b)Indicate outliers' value. |
 | Fig. 2Effects of GA on liver enzymes (a)significant difference from baseline). (A) Effects of GA on serum ALT Level (P=0.077). (B) Effects of GA on serum AST level (P=0.190). (C) Effects of GA on serum ALP level (P=0.211). b)Indicate outliers' value |
 | Fig. 3Effects of GA on renal function (a)significant difference from baseline). (A) Effects of GA on serum urea level (P=0.196). (B) Effects of GA on serum creatinine level (P=0.205). b)Indicate outliers' value. |
ACKNOWLEDGMENTS
We are thankful to the Department of Clinical Chemistry, Central Laboratory Military Hospital for their valuable help in estimating renal and liver functions. We are grateful to Dar Savanna Ltd., Sudan for providing gum arabic (Acacia Senegal) powder. The authors would like to thank the participants and investigators in the study.
Notes
References
1. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008; 86:480–487.



2. Little JA, McGowan VR, Kato GJ, et al. Combination erythropoietin-hydroxyurea therapy in sickle cell disease: experience from the National Institutes of Health and a literature review. Haematologica. 2006; 91:1076–1083.


3. Kaddam L, Fadl-Elmula I, Eisawi OA, et al. Gum Arabic as novel anti-oxidant agent in sickle cell anemia, phase II trial. BMC Hematol. 2017; 17:4.



4. Pandey S, Sharma A, Dahia S, et al. Biochemical indicator of sickle cell disease: preliminary report from India. Indian J Clin Biochem. 2012; 27:191–195.


5. Kotila T, Adedapo K, Adedapo A, Oluwasola O, Fakunle E, Brown B. Liver dysfunction in steady state sickle cell disease. Ann Hepatol. 2005; 4:261–263.


6. Nduka N, Kazem Y, Saleh B. Variation in serum electrolytes and enzyme concentrations in patients with sickle cell disease. J Clin Pathol. 1995; 48:648–651.



7. Yahaya IA. Biochemical features of hepatic dysfunction in Nigerians with sickle cell anaemia. Niger Postgrad Med J. 2012; 19:204–207.

8. Oparinde DP, Oghagbon EK, Okesina AB, Olatunji PO, Ojuawo AO. Role of hepatic enzymes in the biochemical assessment of the severity of sickle cell anemia. Trop Gastroenterol. 2006; 27:118–121.

9. Wright JG, Malia R, Cooper P, Thomas P, Preston FE, Serjeant GR. Protein C and protein S in homozygous sickle cell disease: does hepatic dysfunction contribute to low levels? Br J Haematol. 1997; 98:627–631.


10. Thompson J, Reid M, Hambleton I, Serjeant GR. Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med. 2007; 167:701–708.


11. Aloni MN, Ngiyulu RM, Gini-Ehungu JL, et al. Renal function in children suffering from sickle cell disease: challenge of early detection in highly resource-scarce settings. PLoS One. 2014; 9:e96561.

12. Morgan AG, Serjeant GR. Renal function in patients over 40 with homozygous sickle-cell disease. Br Med J (Clin Res Ed). 1981; 282:1181–1183.



13. Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol. 2010; 25:2123–2127.


14. Agoreyo FO, Nwanze N. Plasma sodium and potassium changes in sickle cell patients. Int J Genet Mol Biol. 2010; 2:14–19.
15. De Ceulaer K, Morgan AG, Choo-Kang E, Wilson WA, Serjeant GR. Serum urate concentrations in homozygous sickle cell disease. J Clin Pathol. 1981; 34:965–969.



17. Rothschild BM, Sienknecht CW, Kaplan SB, Spindler JS. Sickle cell disease associated with uric acid deposition disease. Ann Rheum Dis. 1980; 39:392–395.



18. Morgan AG, De Ceulaer K, Serjeant GR. Glomerular function and hyperuricaemia in sickle cell disease. J Clin Pathol. 1984; 37:1046–1049.



19. Ali BH, Al-Husseni I, Beegam S, et al. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS One. 2013; 8:e55242.

20. Al-Majed AA, Abd-Allah AR, Al-Rikabi AC, Al-Shabanah OA, Mostafa AM. Effect of oral administration of arabic gum on cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol. 2003; 17:146–153.


21. Kaddam L, FdleAlmula I, Eisawi OA, et al. Gum arabic as fetal hemoglobin inducing agent in sickle cell anemia; in vivo study. BMC Hematol. 2015; 15:19.



22. Palmer SM, Kaufman RA, Salamone SJ, et al. Cobas Integra: clinical laboratory instrument with continuous and random-access capabilities. Clin Chem. 1995; 41:1751–1760.


23. Gamal el-din AM, Mostafa AM, Al-Shabanah OA, Al-Bekairi AM, Nagi MN. Protective effect of arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol Res. 2003; 48:631–635.


24. Ali BH, Ziada A, Blunden G. Biological effects of gum arabic: a review of some recent research. Food Chem Toxicol. 2009; 47:1–8.


25. Ross AH, Eastwood MA, Brydon WG, Anderson JR, Anderson DM. A study of the effects of dietary gum arabic in humans. Am J Clin Nutr. 1983; 37:368–375.

26. Ali BH, Beegam S, Al-Lawati I, Waly MI, Al Za'abi M, Nemmar A. Comparative efficacy of three brands of gum acacia on adenine-induced chronic renal failure in rats. Physiol Res. 2013; 62:47–56.


27. Nasir O, Artunc F, Saeed A, et al. Effects of gum arabic (Acacia senegal) on water and electrolyte balance in healthy mice. J Ren Nutr. 2008; 18:230–238.


28. Khalid SA, Musa AM, Saeed AM, et al. Manipulating dietary fibre: Gum arabic making friends of the colon and the kidney. Bioactive carbohydr Diet Fibre. 2014; 3:71–76.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download