INTRODUCTION
MATERIALS AND METHODS
Study design and subjects
 | Fig. 1Flow diagram of study progression.ACT, asthma control test; GINA, Global Initiative for Asthma; FeNO, fractional exhaled nitric oxide; anti-PR3, autoantibody against proteinase-3; anti-Sm, autoantibody against Smith antigen; anti-P0, autoantibody against ribosomal phosphoprotein P0; anti-SSA, autoantibody against Ro/Sjögren syndrome type A antigen; anti-SSB, autoantibody against La/Sjögren syndrome type B antigen; anti-Scl-70, autoantibody against DNA topoisomerase; anti-Jo1, autoantibody against histidyl-tRNA synthetase; anti-U1-SnRNP, autoantibody against U1small nuclear ribonucleoprotein; anti-MPO, autoantibody against myeloperoxidase; anti-TPO, autoantibody against thyroid peroxidase.
|
Pulmonary function and FeNO
Allergen sensitization
Collection and processing of induced Sp and Se
Detection of Abs
Statistical analysis and visualization
RESULTS
Patients
Table
Demographic and clinical characteristics of healthy controls and patients with different severity of asthma

Sp- and Se-Ab concentrations and their associations with clinical parameters
 | Fig. 2Ten types of Sp (panel A) and Se (panel B) Abs in asthmatic patients and healthy volunteers.Sp, sputum; Se, serum; Ab, autoantibody; anti-PR3, autoantibody against proteinase-3; anti-SSB, autoantibody against La/Sjögren syndrome type B antigen; anti-SSA, autoantibody against Ro/Sjögren syndrome type A antigen; anti-Sm, autoantibody against Smith antigen; anti-U1-SnRNP, autoantibody against U1small nuclear ribonucleoprotein; anti-Scl-70, autoantibody against DNA topoisomerase; anti-P0, autoantibody against ribosomal phosphoprotein P0; anti-Jo1, autoantibody against histidyl-tRNA synthetase; anti-MPO, autoantibody against myeloperoxidase; anti-TPO, autoantibody against thyroid peroxidase.
*P < 0.05 indicates statistical significance between 2 groups for the concentration of Ab (Mann-Whitney U test).
|
 | Fig. 3The scatter plots of the correlations between Sp- and Se-Abs with clinical characteristics (the correlations with P > 0.05 were not shown). (A-D) performed partial correlation analysis adjusted by age. (E-H) performed Spearman correlation analysis.Sp, sputum; Se, serum; Ab, autoantibody; ICS, inhaled corticosteroid; BDP, beclomethasone dipropionate; FeNO, fractional exhaled nitric oxide; FI, fluorescence intensity; anti-SnRNP, autoantibody against small nuclear ribonucleoprotein; anti-PR3, autoantibody against proteinase-3; anti-SSA, autoantibody against Ro/Sjögren syndrome type A antigen; anti-Sm, autoantibody against Smith antigen; anti-Scl-70, autoantibody against DNA topoisomerase.
|
The correlation matrix of Abs
 | Fig. 4The correlation matrix of Abs.Sp, sputum; Se, serum; Ab, autoantibody; anti-PR3, autoantibody against proteinase-3; anti-SSB, autoantibody against La/Sjögren syndrome type B antigen; anti-Sm, autoantibody against Smith antigen; anti-U1-SnRNP, autoantibody against U1small nuclear ribonucleoprotein; anti-SSA, autoantibody against Ro/Sjögren syndrome type A antigen; anti-Scl-70, autoantibody against DNA topoisomerase; anti-P0, autoantibody against ribosomal phosphoprotein P0; anti-Jo1, autoantibody against histidyl-tRNA synthetase; anti-MPO, autoantibody against myeloperoxidase; anti-TPO, autoantibody against thyroid peroxidase.
*P < 0.05, †P < 0.01, ‡P < 0.001, a circle represents a correlation between Sp- and Se-Abs regarding 1 type of Ab.
|
Network-based analysis
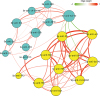 | Fig. 5The network-based analysis for the associations between Abs in Sp and Se. The network graph was constructed with the 20 Abs. Each Ab is represented in the graph by a specific node who's color represents a specific cluster and the size of the node is proportional to the sum of the edges that connect to them. Edges between nodes represent a statistically significant association (P < 0.05). The edge's thickness represents the strengths of their association (correlation coefficient, Spearman's correlation). The correlation coefficient is represented by color, with green and purple indicating positive and negative relationships, respectively.Ab, autoantibody; Sp, sputum; Se, serum; anti-PR3, autoantibody against proteinase-3; anti-Sm, autoantibody against Smith antigen; anti-P0, autoantibody against ribosomal phosphoprotein P0; anti-SSA, autoantibody against Ro/Sjögren syndrome type A antigen; anti-SSB, autoantibody against La/Sjögren syndrome type B antigen; anti-Scl-70, autoantibody against DNA topoisomerase; anti-Jo1, autoantibody against histidyl-tRNA synthetase; anti-U1-SnRNP, autoantibody against U1small nuclear ribonucleoprotein; anti-MPO, autoantibody against myeloperoxidase; anti-TPO, autoantibody against thyroid peroxidase.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download