INTRODUCTION
Human epidermal growth factor receptor-2 (HER2) is an adverse risk factor for relapsed breast cancer [
1]. Trastuzumab, a monoclonal antibody targeting HER2, has dramatically improved the overall survival (OS) and disease-free survival (DFS) in patients with HER2 overexpression in both adjuvant and palliative settings [
234]. The administration of trastuzumab has become the standard treatment in patients with HER2-positive breast cancers [
5].
HER2 overexpression is reportedly associated with increased risk of brain metastasis both as the site of the first (4.3% vs. 0.4%) and eventual relapse [
6]. Autopsy data show that the incidence hazard ratio (HR) of central nervous system (CNS) metastasis is higher (up to 50%) in HER2-positive than in HER2-negative breast cancer [
7]. A retrospective study on 9,524 patients with early breast cancer identified HER2 positivity as a clear risk factor for the development of CNS relapse [
8]. However, the exact biological mechanism for the tendency of HER2-positive cancer cells to metastasize to the CNS remains to be completely elucidated.
In addition, more CNS relapses have been observed in patients treated with trastuzumab for metastatic breast cancers [
910]. In the adjuvant setting, a meta-analysis of randomized controlled trials comparing adjuvant trastuzumab and no trastuzumab administration in HER2-positive breast cancers noted that the CNS as the first site of failure was more common in patients receiving trastuzumab [
11]. Moreover, a population-based study reported that adjuvant trastuzumab treatment was associated with higher CNS failure rate [
12]. However, whether the increased CNS relapse is correlated with adjuvant trastuzumab remains controversial [
13].
Among the patients with HER2-positive breast cancers, those with hormonal receptor-negative tumors are known to have more CNS failures than those with hormonal receptor-positive tumors [
14]. However, most studies on HER2-positive tumors have included both types of tumors with their different molecular subtypes; thus, the interpretation is somewhat complicated.
This study evaluated the outcomes of surgery followed by postoperative radiotherapy (RT) in Korean patients with breast cancer having HER2-enriched subtype and compared the risk of CNS failures according to the administration of adjuvant trastuzumab.
DISCUSSION
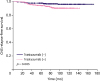
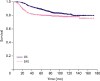
In this study, we observed that CNS relapse-free survival was associated with adjuvant trastuzumab in patients with HER2-enriched subtype of breast cancer. The administration of adjuvant trastuzumab was mainly determined according to the treatment period; however, the number of patients with nodal involvement and lymphatic invasion in the trastuzumab (+) group was higher than that in the trastuzumab (−) group. After adjusting these covariates using the multivariate analysis, the adjuvant trastuzumab remained the only risk factor associated with higher CNS relapse rate.
HER2-positive breast cancer is a disease with distinct clinicopathological features and accounts for 15–20% of all invasive breast cancers, and it is characterized by a particularly aggressive course [
15]. HER2-positive tumors have a higher risk of CNS relapse compared with HER2-negative tumors [
6]. In addition, among the HER2-positive tumors, the HER2-enriched subtype presents with a higher risk of CNS relapse compared with HER2-/hormonal receptor-positive tumors. This was confirmed by Lim et al. who reported that the CNS relapse rate was higher in the HER2-enriched subtype than in HER2-/hormonal receptor-positive tumors (10-year rate, 3.5% vs. 0.0%,
p = 0.001) [
14]. In a population-based study of HER2-positive patients with breast cancer, hormonal receptor negativity was the independent predictive factor for CNS relapse with HR of 5.4 [
12]. Given these observations, 749 patients from eight institutions in Korea were included, and the surgical outcomes followed by postoperative RT in HER2-enriched subtype evaluated. Consequently, we observed that the administration of adjuvant trastuzumab was associated with an increased risk of CNS relapse.
Regarding the correlation between trastuzumab and CNS relapse, 523 patients with metastatic breast cancer were enrolled in two clinical trials of the first-line trastuzumab therapy, which revealed a 10% incidence of isolated CNS progression [
16]. Nearly one-third of patients with HER2-positive metastatic breast cancer are now developing CNS disease despite receiving trastuzumab-based therapy [
917]. In contrast, the incidence of CNS metastasis in a historical series was only 10–16% [
1819]. These increased incidences of CNS events not only reflect the inherent behavior of HER2-positive tumors but also improved survival by trastuzumab treatment in these patients, allowing more CNS events to become clinically evident before death [
10].
However, whether “adjuvant” trastuzumab is associated with increased risk of CNS failure remains controversial. Musolino et al. [
12] evaluated the risk of CNS failure in a population-based cancer registry and noted that CNS failure, either as the first or a subsequent recurrence, was significantly more common in trastuzumab-treated patients with HER2-positive breast cancer based on the multivariate analysis. They postulated that the improved systemic control and OS because of trastuzumab may reveal the silent CNS failure. In a combined analysis of the NASBP B-31 and NCCTG N9831 trials, Romond et al. [
3] reported that the incidence of isolated brain metastases as the first event was higher in the trastuzumab group. In addition, a meta-analysis of randomized controlled trials revealed that the adjuvant trastuzumab group had a higher CNS failure rate compared with the observation group [
11].
Conversely, in the NASBP B-31 trial, the incidence of brain metastases as the first or subsequent event was similar between the trastuzumab and control groups [
3]. Therefore, they explained that increased brain metastases as the first event in the trastuzumab group were due to early non-CNS failures in the control group. In addition, a retrospective analysis of the HERceptin Adjuvant (HERA) trial revealed that CNS relapse was not increased in trastuzumab-treated patients [
13]. Pestalozzi et al. [
13] observed that the frequency of CNS relapse at the first DFS event was comparable in patients receiving and not receiving trastuzumab.
Regarding these conflicting observations, most investigators agree that the improved survival because of trastuzumab treatment contributes to the paradoxical increase of CNS relapses as the first sites of failure. Although prolonging OS, the penetration of trastuzumab into the intact blood–brain barrier is very limited due to its large molecular weight and limited efficacy in controlling micrometastasis in the CNS [
20]. However, if OS is “relatively” limited in the control group, a significant proportion of CNS relapses may eventually be masked, resulting in increased CNS relapses as subsequent failures in the trastuzumab group. Conversely, if OS is maintained “relatively” enough to reveal the silent CNS failures in the control group, the incidence of CNS failures as subsequent events seems to be proportional between the 2 groups.
Most of these studies included both HER2-/hormonal receptor-positive and HER2-/hormonal receptor-negative tumors. In the present study, only HER2-enriched tumors were analyzed, and cumulative CNS relapses were counted. Consequently, in the multivariate analysis, administration of adjuvant trastuzumab was the only significant prognosticator of a higher CNS relapse rate. As previously mentioned, the number of patients with nodal involvement, high-grade tumor, and lymphatic invasion in the trastuzumab (+) group was higher, thus allowing for a higher risk of CNS failure. However, the similar survival rates between the trastuzumab (+) and trastuzumab (−) groups suggest the survival benefit of adjuvant trastuzumab in high-risk patients, although the difference was not statistically significant. This finding is consistent with the hypothesis that trastuzumab improves the survival but not the CNS control, resulting in increased CNS relapses. Although nodal involvement and lymphatic invasion were associated with CNS relapses on univariate analysis, statistical significance was not observed in the multivariate analysis. Lymphovascular invasion was also a strong prognosticator of both DFS and OS. Recently, Hamy et al. [
21] noted that the magnitude of its effect was dependent on the molecular subtype with the greatest HR reported for the HER2-positive breast cancer. A recent study using the Surveillance, Epidemiology, and End Results database reported that HER2-enriched subtype showed the highest incidence of brain metastasis among 206,913 patients with breast cancer. The HER2-enriched and triple-negative subtypes with multiple extracranial metastases (bone, liver, and lung) showed high incidences of brain metastasis (28.0, and 30.8%, respectively). The authors concluded that patients with HER2-enriched and triple-negative subtypes having visceral metastasis should be closely monitored in order to contribute to early detection of brain metastasis [
22]. Further research is warranted to elucidate the possible contribution of other risk factors to the occurrence of CNS relapse and to identify potential candidates for close surveillance, including brain imaging. Several ongoing prospective clinical trials are testing various HER2-targeting agents combined with whole-brain RT or stereotactic radiosurgery in patients with CNS failure (
Table 5), and their findings are expected to recommend a reasonable combination strategy to improve the clinical outcomes in the near future.
Table 5
Representative ongoing prospective clinical trials on brain metastases from HER2-positive breast cancer

|
Agent |
Phase |
NCT No. |
Study design |
|
Lapatinib |
2 |
01622868
|
WBRT or SRS +/− lapatinib |
|
Tucatinib |
2 |
02614794
|
Tucatinib + capecitabine/trastuzumab vs. capecitabine/trastuzumab |
|
Pertuzumab/trastuzumab |
2 |
02536339
|
Intravenous pertuzumab + trastuzumab following WBRT or SRS |
|
1 |
02598427
|
Intrathecal pertuzumab + trastuzumab |
|
Trastuzumab |
1 |
02571530
|
Super-selective intra-arterial trastuzumab |
|
Tesevatinib |
1/2 |
02154529
|
Tesevatinib + trastuzumab |
|
T-DM1 |
1/2 |
03190967
|
T-DM1 vs. T-DM1 + temozolomide following SRS |
|
2 |
03203616
|
T-DM1 |

One of the limitations of this study is its retrospective study design. Although the administration of trastuzumab was determined mainly according to the treatment period, potential biases may be present. As previously mentioned, the number of patients in the trastuzumab (+) group with nodal involvement and lymphovascular invasion, the significant prognosticators of CNS relapse-free survival in the univariate analysis, was higher. After adjusting for covariates including these two variables, adjuvant trastuzumab administration remained the only prognosticator with statistical significance; however, the imbalances of tumor characteristics may not be fully controlled in the multivariate analysis. In addition, although all patients underwent systemic chemotherapy and local treatment such as surgery followed by RT, differences in chemotherapeutic regimens and RT details may contribute to the heterogeneity.
In conclusion, adjuvant trastuzumab was associated with higher CNS failure rate in Korean patients with breast cancer with HER2-enriched subtype. These increased incidences of CNS events could reflect the limited penetration of trastuzumab into the blood–brain barrier and improved survival, which allowed more CNS events to become clinically evident before death. Close monitoring and reasonable approaches, such as CNS-penetrating HER2 blockades combined with current standard therapy, could contribute to improving the intracranial tumor control and the quality of life in patients with CNS metastasis from HER2-enriched breast cancer.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download