INTRODUCTION
METHODS
Inclusion and exclusion criteria
Variable declaration
Statistical analysis
RESULTS
Patient demographics and tumor characteristics according to HR status
Table 1
Characteristics of 181.108 patients with breast cancer

Univariate and multivariate analyses based on competing risk regression model
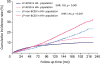 | Figure 1Cumulative incidence of LP-BCSD and LP-non-BCSD for HR+ and HR− breast cancer. HR+ breast cancer had more LP-BCSD in the whole cohort compared to HR− breast cancer (SHR, 1.18; 95% CI, 1.13–1.24; p < 0.001). Moreover, HR+ was associated with more LP-non-BCSD (SHR, 1.41; 95% CI, 1.35–1.47; p < 0.001). The risk of LP-BCSD was exceeded by that of LP-non-BCSD in the HR+ and HR− subgroups. The curves were plotted using the Gray method.LP-BCSD = later period breast cancer-specific death; LP-non-BCSD = later period non-breast cancer-specific death; HR = hormone receptor; SHR = subdistribution hazard ratio; CI = confidence index.
|
Table 2
LP-BCSD and non-LP-BCSD in univariate and multivariate analysis

Stratified analysis for LP-BCSD and LP-non-BCSD according to HR status
 | Figure 2Forest plot of the subgroup analysis for LP-BCSD and LP-non-BCSD according to HR status. (A) The forest plot of subgroup (HR+ vs. HR−) analysis for LP-BCSD. In the low-stage subgroups (including T1, N0 and stage I), patients with HR+ tumors had less LP-BCSD. (B) The forest plot of subgroup (HR+ vs. HR−) analysis for LP-non-BCSD; there was no difference in all 4 age subgroups.LP-BCSD = later period breast cancer-specific death; LP-non-BCSD = later period non-breast cancer-specific death; HR = hormone receptor; IDC = infiltrating duct carcinoma; ILC = infiltrating lobular carcinoma; SHR = subdistribution hazard ratio; CI = confidence index.
|
 | Figure 3Impact of HR status on LP-BCSD and LP-non-BCSD by subgroup analysis according to age subgroups. (A) In the 20–40 year-old subgroup, patients with HR+ breast cancer had more LP-BCSD than patients with HR− breast cancer (SHR, 2.30; 95% CI, 2.02–2.62; p < 0.001). Moreover, the risk of LP-BCSD was higher than LP-non-BCSD in both the HR+ and HR− subgroups. (B) In the 40–60 year-old subgroup, patients with HR+ breast cancer had more LP-BCSD than patients with HR− breast cancer (SHR, 1.18; 95% CI, 1.11–1.26; p < 0.001). Moreover, the risk of LP-BCSD was higher than LP-non-BCSD in both the HR+ and HR− subgroups. (C) In the 60–70 year-old subgroup, the risk of LP-BCSD in HR+ breast cancer was similar to that of HR− breast cancer (SHR, 0.98; 95% CI, 0.88–1.09; p = 0.644). Moreover, the risk of LP-BCSD was exceeded by that of LP-non-BCSD in both the HR+ and HR− subgroups. (D) In the 70–80 year-old subgroup, the risk of LP-BCSD in the HR+ subgroup has a decreasing trend compared to that of the HR− subgroup, without a statistically significant difference (SHR, 0.93; 95% CI, 0.81–1.05; p = 0.241). The risk of LP-BCSD had been exceeded by LP-non-BCSD in both the HR+ and HR− subgroups at the beginning of follow up.LP-BCSD = later period breast cancer-specific death; LP-non-BCSD = later period non-breast cancer-specific death; HR = hormone receptor; SHR = subdistribution hazard ratio; CI = confidence index.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download