This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The Z0011 trial showed that axillary lymph node dissection (ALND) can be safely avoided in breast cancer patients with low nodal burden (LNB). ALND can be performed in patients with high nodal burden (HNB). We aimed to determine whether HNB in early breast cancer patients can be predicted preoperatively to avoid sentinel lymph node biopsy (SLNB).
Methods
Early invasive breast cancer patients (cT1-2cN0) were retrospectively reviewed. We excluded patients with neoadjuvant chemotherapy and incomplete data. The patients were divided into the following groups based on surgical histology: no positive (N0), LNB, and HNB, defined as 0, 1–2, and ≥ 3 metastatic lymph nodes (LNs), respectively. Of the patients with metastatic nodal disease, only those with ALND were included in the analysis. Clinical, radiological, and histological parameters were evaluated using logistic regression analysis as predictors of HNB versus LNB and N0 combined.
Results
Of the 1,298 included patients, 832 (64.1%), 286 (22.0%), and 180 (13.9%) had N0, LNB, and HNB, respectively. Univariate logistic regression analysis revealed that sonographic features of breast tumor size (p < 0.0001), number of abnormal LNs (p < 0.0001), cortical thickness (p = 0.0002), effacement of the fatty hilum (p < 0.0001), and needle biopsy being performed (p < 0.0001) were indicators of HNB. Breast tumor grade (p = 0.0001) and human epidermal growth factor receptor 2 status (p = 0.0262) were also statistically significant. Among these significant features, multivariable stepwise logistic regression showed that the number of abnormal LNs is the sole independent predictor of HNB (p < 0.0001, area under the curve = 0.774). The positive predictive value of HNB in patients with ≥ 4 abnormal LNs was 92.9%.
Conclusion
The detection of ≥ 4 abnormal LNs on ultrasound can help to identify HNB patients who require upfront ALND and thus avoid SLNB.
Keywords: Breast neoplasms, Lymph node dissection, Sentinel lymph node biopsy
INTRODUCTION
The Z0011 trial has shown that early breast cancer patients with low nodal burden (LNB) can be spared axillary lymph node dissection (ALND) with no difference in survival outcome [
1]. ALND can be reserved for patients with high nodal burden (HNB), defined as having 3 or more metastatic lymph nodes (LNs), detected via sentinel lymph node biopsy (SLNB) in the Z0011 trial. HNB patients accounted for 21% of the ALND arm cohort in the Z0011 trial [
2].
While SLNB remains the gold standard for the assessment of axillary nodal burden [
3], it may be an unnecessary procedure in the HNB group who require an ALND if we can identify this subgroup preoperatively [
4]. However, only a limited amount of data is available to distinguish the HNB group preoperatively.
We aimed to determine the clinical, radiological, or pathological features that could be used to distinguish patients with HNB preoperatively from the entire cohort of early invasive breast cancer patients (cT1-2cN0) so that they can undergo an upfront ALND and avoid SLNB.
METHODS
Newly diagnosed invasive breast cancer female patients (cT1-2N0M0), based on histological biopsy, admitted at the KK Women's and Children's Hospital, Republic of Singapore, from January 2007 to March 2017 were retrospectively reviewed. We excluded patients with pure ductal carcinoma in situ, with recurrent breast cancers, incomplete data, and who received neoadjuvant chemotherapy.
All patients in this study underwent an axillary ultrasound evaluation in addition to their routine mammogram and breast ultrasound examinations. If any abnormal LN was detected on ultrasound, a needle biopsy (fine needle aspiration or core needle biopsy) of the most suspicious-looking LN would be offered. In our institution, a LN was considered abnormal if any of the following features was present: cortical thickness of more than 3 mm, eccentric cortical thickening of more than 2 mm, or marked fatty hilar effacement.
In accordance with our local practice, patients with negative axillary ultrasound or needle biopsy results would need to undergo SLNB. On the contrary, patients with positive needle biopsy or SLNB results would need to undergo ALND. We also included patients with an ALND but with negative nodal burden. Most of these patients had ALND because of a failed SLNB.
These eligible patients were then divided into the following groups based on surgical axillary histology: no positive LNs (N0), with 1–2 metastatic LNs (LNB), and with ≥ 3 metastatic LNs (HNB). We excluded patients with histologically proven metastatic axillary disease but did not undergo ALND, as the true status of axillary nodal involvement may not be accurately reflected in these cases.
The preoperative clinical, radiological, and pathological parameters of the HNB subgroup were compared with those of LNB and N0 group combined. The selected cutoff age was 50 years old based on the National Comprehensive Cancer Network definition of early-onset breast cancer. Radiological parameters included sonographic breast tumor size, presence or absence of tumor multifocality, number of abnormal LNs, maximum cortical thickness, and the presence of marked fatty hilum effacement. If these data were not available on the radiological reports, the ultrasound images would be reviewed by a dedicated breast radiologist to complete the data. Pathological parameters were derived from the results of patients' needle or excision biopsy of the breast tumor and were analyzed on the basis of histological subtypes, tumor grade, and receptors. Histological subtypes were classified on the basis of main histological subtypes with others comprising of tubular, cribriform, and adenosquamous, etc.
Statistical analysis
Statistical analysis was performed using SAS V9.4 (SAS Inc., Cary, USA). Categorical clinical, radiological, and histological parameters were expressed as frequency counts and percentages; continuous variables were expressed as mean/median and standard deviation/range. Categorical variables between the HNB group and the N0 and LNB groups were compared using the Pearson χ2 test. Univariate logistic regression was performed to investigate clinical, radiological, and histological variables as predictors of HNB versus the combined N0 and LNB groups. Variables significant in univariate analysis at p ≤ 0.05 were entered into a multivariable stepwise logistic regression analysis with p ≤ 0.05 being defined as statistically significant.
This study was approved by the SingHealth Centralised Institutional Review Board (CIRB Ref: 2017/2077), and the need for informed consent was waived.
RESULTS
A total of 1,382 patients were cT1-2cN0. Approximately 1,343 patients were available for analysis after excluding 39 who had undergone neoadjuvant chemotherapy and had incomplete data (
Figure 1). The patients with incomplete data included those underwent preoperative breast imaging or biopsy at other institutions but were subsequently examined at our institution for treatment.
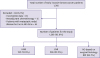 | Figure 1
Flowchart of the patients in the study.
N0 = no positive lymph nodes; ALND = axillary lymph node dissection; HNB = high nodal burden; LNB = low nodal burden.

|
Of the 1,343 patients, 45 with metastatic nodal disease were further excluded as they did not undergo ALND. Of the remaining 1,298 patients, 832 (64.1%), 286 (22.0%), and 180 (13.9%) had N0, LNB, and HNB, respectively. Among these 1,298 patients, 832 (64.1%), 189(14.6%), 97 (7.5%), 47 (3.6%), and 133 (10.2%) had 0, 1, 2, 3, and ≥ 4 metastatic LNs, respectively.
Median/mean age of all 1,298 patients was 53/53.8 years (range, 21–95 years). The median/mean tumor size, based on ultrasound, was 19.0/20.2 mm (range, 0.0–50.0 mm). Preoperatively, 86.9% of patients had invasive ductal cancer and 28.4% had grade III tumor, based on diagnostic biopsy results. Approximately 79.7% of patients had estrogen receptor-positive breast cancer, while 69.0% of patients had progesterone receptor-positive breast cancer. About 17.9% of patients had human epidermal growth factor receptor 2 (HER2)-positive breast cancer.
The sensitivity and specificity of ultrasound axillary were 45.1% and 92.2%, respectively. Needle biopsy of the axillary LNs was performed in 242 (18.6%) patients. The sensitivity and specificity of needle biopsy were 77.5% and 98.2%, respectively.
In patients with N0 based on SLNB, we also took into consideration the number and histological status of any incidental LNs, which were harvested as well. The median/mean number of LNs harvested in this group were 3/2.83 (range, 1–13).
Moreover, 13 (1.0%) patients with negative nodal burden underwent ALND. The majority of patients underwent ALND because of failure to identify the sentinel LNs. These patients were included in the N0 group for analysis.
The median/mean number of LNs harvested during ALND for LNB and HNB were 16.5/16.8 and 19.0/19.2, respectively.
On final histology, 584 (45.0%), 675 (52.0%), 39 (3.0%), and 0 (0%) patients had pT1, pT2, pT3, and pT4, respectively. With respect to LN status, 832 (64.1%), 333 (25.7%), 95 (7.3%), and 38 (2.9%) patients had pN0, pN1, pN2, and pN3 respectively.
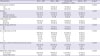
All radiologically studied parameters exhibited significant differences in frequency distribution between the HNB group and the combined N0 and LNB group (
Table 1). In the univariate logistic regression analysis, significant radiological risk factors for HNB were larger tumor size (
p < 0.0001), needle biopsy being performed (
p < 0.0001), increased number of abnormal LNs (
p < 0.0001), increased cortical thickness (
p = 0.0002), and effacement of the fatty hilum (
p < 0.0001).
Table 1
Ultrasound imaging parameters of patients with HNB, LNB, and N0 with analysis between N0 and LNB combined versus HNB
|
Characteristics |
N0 (n = 832) |
LNB (n = 286) |
N0 and LNB combined (n = 1,118) |
HNB (n = 180) |
p-value*
|
|
Tumor size on US (mm) |
|
|
|
|
< 0.0001 |
|
≤ 20 |
501 (60.2) |
150 (52.4) |
651 (58.2) |
72 (40.0) |
|
> 20 to ≤ 50 |
331 (39.8) |
136 (47.6) |
467 (41.8) |
108 (60.0) |
|
Focality |
|
|
|
|
0.0001 |
|
Single |
640 (76.9) |
180 (63.0) |
820 (73.4) |
108 (60.0) |
|
Multiple ipsilateral |
158 (19.0) |
95 (33.2) |
253 (22.6) |
67 (37.2) |
|
Multiple contralateral |
34 (4.1) |
11 (3.8) |
45 (4.0) |
5 (2.8) |
|
Axilla ultrasound |
|
|
|
|
< 0.0001 |
|
Normal |
767 (92.2) |
192 (67.1) |
959 (85.8) |
63 (35.0) |
|
Abnormal |
65 (7.8) |
94 (32.9) |
159 (14.2) |
117 (65.0) |
|
Needle biopsy performed |
|
|
|
|
< 0.0001 |
|
Yes |
55 (6.6) |
90 (31.5) |
145 (13.0) |
97 (53.9) |
|
No |
777 (93.4) |
196 (68.5) |
973 (87.0) |
83 (46.1) |
|
No. of abnormal LN on US |
|
|
|
|
< 0.0001 |
|
0 |
767 (92.1) |
183 (64.0) |
950 (85.0) |
62 (34.4) |
|
1 |
49 (5.9) |
79 (27.6) |
128 (11.4) |
45 (25.0) |
|
2 |
13 (1.6) |
20 (7.0) |
33 (3.0) |
37 (20.6) |
|
3 |
3 (0.4) |
3 (1.0) |
6 (0.5) |
23 (12.8) |
|
≥ 4 |
0 (0) |
1 (0.4) |
1 (0.1) |
13 (7.2) |
|
Maximum cortical thickness of abnormal LN (mm) |
|
|
|
|
< 0.0001 |
|
< 3 |
830 (99.8) |
284 (99.3) |
1,114 (99.6) |
172 (95.6) |
|
3–4 |
0 (0) |
2 (0.7) |
2 (0.2) |
0 (0) |
|
> 4 |
2 (0.2) |
0 (0) |
2 (0.2) |
8 (4.4) |
|
Effacement of fatty hilum |
|
|
|
|
< 0.0001 |
|
No or partial |
826 (99.3) |
248 (86.7) |
1,074 (96.1) |
127 (70.6) |
|
Marked effacement |
6 (0.7) |
38 (13.3) |
44 (3.9) |
53 (29.4) |

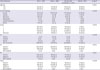
There were no significant differences in age distribution between the HNB group and the combined N0 and LNB group (
Table 2). Histological parameters exhibiting differences in frequency distribution between the HNB group and combined N0 and LNB group were breast tumor histological type, grade, lymphovascular invasion (LVI), and HER2 (
Table 2). In the univariate logistic regression analysis, significant histological risk factors for HNB were tumor grade (
p = 0.0001), LVI (
p = 0.0038), and HER2 status (
p = 0.0262).
Table 2
Clinical and diagnostic biopsy parameters of patients with HNB, LNB and N0 with analysis between N0 and LNB combined versus HNB
|
Clinical features |
N0 (n = 832) |
LNB (n = 286) |
N0 and LNB combined (n = 1,118) |
HNB (n = 180) |
p-value*
|
|
Age (yr) |
|
|
|
|
0.1424 |
|
≤ 50 |
355 (42.7) |
114 (39.9) |
469 (41.9) |
86 (47.8) |
|
> 50 |
477 (57.3) |
172 (60.1) |
649 (58.1) |
94 (52.2) |
|
Tumor histology |
|
|
|
|
0.0033 |
|
Ductal |
701 (84.3) |
269 (94.0) |
970 (86.8) |
158 (87.8) |
|
Lobular |
40 (4.8) |
9 (3.2) |
49 (4.4) |
17 (9.4) |
|
Mucinous |
56 (6.7) |
4 (1.4) |
60 (5.4) |
0 (0) |
|
Metaplastic |
9 (1.1) |
0 (0) |
9 (0.8) |
1 (0.6) |
|
Invasive papillary |
7 (0.8) |
0 (0) |
7 (0.6) |
1 (0.6) |
|
Others |
19 (2.3) |
4 (1.4) |
23 (2.0) |
3 (1.6) |
|
Grade |
|
|
|
|
0.0160 |
|
I |
149 (17.9) |
36 (12.6) |
185 (16.5) |
14 (7.8) |
|
II |
344 (41.4) |
126 (44.1) |
470 (42.1) |
81 (45.0) |
|
III |
213 (25.6) |
95 (33.2) |
308 (27.5) |
61 (33.9) |
|
Unknown |
126 (15.1) |
29 (10.1) |
155 (13.9) |
24 (13.3) |
|
LVI |
|
|
|
|
0.0003 |
|
Present |
1 (0.1) |
6 (2.0) |
7 (0.6) |
6 (3.3) |
|
Possible |
0 (0) |
1 (0.4) |
1 (0.1) |
2 (1.1) |
|
Absent |
2 (0.2) |
0 (0) |
2 (0.2) |
0 (0) |
|
Not mentioned |
829 (99.7) |
279 (97.6) |
1,108 (99.1) |
172 (95.6) |
|
ER |
|
|
|
|
0.8143 |
|
Positive |
665 (79.9) |
228 (79.7) |
893 (79.9) |
141 (78.3) |
|
Negative |
166 (20.0) |
58 (20.3) |
224 (20.0) |
39 (21.7) |
|
Not available |
1 (0.1) |
0 (0) |
1 (0.1) |
0 (0) |
|
PR |
|
|
|
|
0.7215 |
|
Positive |
586 (70.5) |
188 (65.7) |
774 (69.2) |
121 (67.2) |
|
Negative |
244 (29.3) |
98 (34.7) |
342 (30.6) |
59 (32.8) |
|
Not available |
2 (0.2) |
0 (0) |
2 (0.2) |
0 (0) |
|
HER2 |
|
|
|
|
0.0095 |
|
Positive |
116 (13.9) |
69 (24.2) |
185 (16.5) |
47 (26.1) |
|
Negative |
613 (73.7) |
190 (66.4) |
803 (71.8) |
114 (63.3) |
|
Equivocal |
91 (10.9) |
27 (9.4) |
118 (10.6) |
19 (10.6) |
|
Not available |
12 (1.5) |
0 (0) |
12 (1.1) |
0 (0) |

In the multivariable stepwise selection regression analysis, the number of abnormal LNs detected on ultrasound was the only significant independent predictor of HNB (
p < 0.0001) (
Table 3). The odds ratios (95% confidence interval) for 1, 2, 3, and 4 abnormal LNs relative to N0 were 5.4 (3.5–8.3), 17.2 (10.1–29.3), 58.7 (23.1–150), and 199 (25.6, not applicable), respectively, with significant difference (
p < 0.0001). The area under the receiver operating characteristic curve was 0.774 (
Figure 2). Approximately 92.9% (13/14) of patients with 4 or more abnormal LNs had HNB, with an associated sensitivity, specificity, positive predictive value (PPV), and negative predictive value of 7.2%, 99.9%, 92.9%, and 87.0%, respectively.
Table 3
The p-value summary of univariate and multivariable stepwise logistic regression analyses for statistically significant radiological and histological parameters as predictors of HNB

|
Radiological and histological parameters |
Univariate |
Multivariable stepwise |
|
Radiological |
|
|
|
No. of abnormal LNs on ultrasound |
< 0.0001 |
< 0.0001 |
|
Needle biopsy performed |
< 0.0001 |
NS |
|
Effacement of fatty hilum |
< 0.0001 |
NS |
|
Breast tumor size measured on ultrasound |
< 0.0001 |
NS |
|
Reported abnormal LN maximum cortical thickness |
0.0002 |
NS |
|
Histological |
|
|
|
Tumor grade |
0.0001 |
NS |
|
LVI |
0.0038 |
NS |
|
HER2 |
0.0262 |
NS |

 | Figure 2
ROC curve of number of abnormal LNs seen on axillary ultrasound as a predictor of HNB. The AUC operating characteristic curve was 0.774.
ROC = receiver operating characteristic; LN = lymph node; HNB = high nodal burden; AUC = area under the curve; Sens = sensitivity; Spec = specificity; PPV = positive predictive value; NPV = negative predictive value.

|
DISCUSSION
Our study showed that there was a significant difference in the results of preoperative axillary ultrasound and some histological features between patients with N0 and LNB and those with HNB. Of these factors, the number of abnormal LNs detected on ultrasound was the factor most predictive of HNB. Our study represents one of the few largest series attempting to identify preoperative factors predictive of this subgroup. On the basis of both statistical and clinical considerations, ≥ 4 abnormal LNs detected on ultrasound was determined as the sole independent predictive factor to determine whether an upfront ALND should be performed or not.
Our histology findings were similar to those reported in the literature, with higher grade and HER2 positivity generally being associated with a poorer prognosis [
5]. In another study [
6] involving those patients included in the Z0011 trial clinical pathway, histological factors such as HER2 status and tumor grade were not considered as predictors of ALND. However, this study only compared the LNB and HNB subgroups and did not include the N0 group in their analysis in contrast with our study. By including patients with N0 status in our analysis, we could preoperatively distinguish HNB patients from the entire cohort of invasive early breast cancer patients who were cT1-2cN0, thus reflecting “real world” data. Additionally, in their study [
6], not all patients underwent preoperative axillary ultrasound, and ALND was not performed on all node-positive patients, which could have determined their true nodal status.
Although LVI was statistically significant in our study, it was not mentioned in many of our patients' preoperative breast cancer biopsy reports. This is not surprising, as assessment of biopsy-based LVI is known to be challenging and frequently discordant with final surgical histology [
7].
Despite histological factors being statistically significant, the number of abnormal LNs detected on ultrasound was overwhelmingly the most important predictive factor of HNB.
The role of axillary ultrasound evaluation has become controversial in the post Z0011 trial era. Before the Z0011 trial, axillary ultrasound played an important role in detecting abnormal LNs. The most suspicious LN would often be subjected to a percutaneous biopsy. If a single LN was proven metastatic, the patient would then undergo an ALND, thus avoiding SLNB.
Post Z0011 trial, the threshold for performing an ALND was higher. As a result, some researchers [
89] argued that performing an axillary ultrasound in these Z0011 trial eligible patients would subject patients with LNB to a percutaneous biopsy and exclude them from the Z0011 pathway for trial of axillary preservation. Although patients with positive percutaneous biopsy tended to have HNB [
10], it has been reported that up to half of these Z0011 eligible patients with positive needle biopsy could still qualify for Z0011 pathway and avoid an ALND [
811]. SLNB was also more accurate than ultrasound assessment; thus, it is unnecessary to perform axillary ultrasound.
However, performing axillary ultrasound evaluations had some advantages. It is a non-invasive examination, which can be performed quickly in the same setting as the breast ultrasound. It increases the confidence that the negative SLNB result is accurate if no abnormal LNs were found on sonography. Determining the axillary status before surgery also helps in the selection of patients for neoadjuvant chemotherapy. The SOUND trial (Sentinel node vs Observation after axillary UltraSouND)[
12] is an ongoing study assessing the feasibility of omitting SLNB in early breast cancer based on axillary ultrasound findings.
Our study found that several individual ultrasound features were significantly associated with HNB. Of the sonographic features, having 4 or more abnormal LNs on ultrasound was most predictive of HNB as it had the highest PPV—almost 93%. Another study [
11] also found that the number of sonographically abnormal LNs in needle biopsy node-positive Z0011 eligible patients was important and could be used to distinguish the difference between patients with HNB and those with LNB in that specific subgroup. Hence, these findings suggest that documenting the number of abnormal axillary LNs detected on ultrasound may be paramount.
To the best of our knowledge, the number of abnormal LNs detected on ultrasound is not universally reported worldwide in many imaging centers. This study could potentially change the axillary ultrasound imaging practice. The role of ultrasound in the post Z0011 trial era is evolving. Instead of advocating against the use of axillary ultrasound in these Z0011 eligible patients, we could modify the way ultrasound was previously conducted, by actively imaging and including the number of abnormal LNs detected in the ultrasound reports, with a maximum of up to 4 LNs to be documented, as demonstrated in our study. This practice would be in contrast to the pre Z0011 trial practice of performing ultrasound examination, i.e., imaging only the most suspicious LN as a positive LN would have mandated an ALND in the past.
In patients with 4 or more abnormal LNs detected on ultrasound, the probability of HNB was very high. This finding suggests that these patients may undergo an upfront ALND. Alternatively, a biopsy can be performed to examine the most suspicious LN, and these patients should undergo neoadjuvant chemotherapy instead, resulting in a change in management. In patients with 3 or fewer abnormal LNs detected on ultrasound, the majority (87.0%) would be eligible for axillary preservation. This is in accordance with available literature showing that Z0011 eligible patients with up to 2 abnormal LNs detected on ultrasound can still undergo an SLNB and have axillary preservation if SLNB showed LNB [
13]. This group of patients may not require percutaneous biopsy in the post Z0011 trial era. Similarly, for patients with no abnormal LNs detected on ultrasound, around 93.9% would qualify for axillary preservation. As such, another implication from our study is that in Z0011 eligible patients, percutaneous biopsy could be performed only in patients with 4 or more abnormal LNs, setting a more selective new criteria for percutaneous biopsy.
The preoperative identification of these Z0011 eligible patients with HNB will help to avoid unnecessary SLNB, its accompanying costs [
14], and complications associated with mapping agents, including radioactivity from a radiocolloid agent and rare but serious anaphylaxis from blue dye [
15]. The operative time was also reduced as these patients could proceed to an upfront ALND instead of undergoing SLNB followed by ALND.
In the post-Z0011 era, there has been a declining trend in performing intraoperative frozen section of the sentinel LN [
16]. As a result, HNB patients may need to undergo another operation for ALND when the histology results are available at a later date. The identification of these HNB patients preoperatively could potentially avoid the need for additional operation.
Our study had some strengths. The patients in this study underwent a routine preoperative axillary ultrasound; hence, we were able to obtain a large and comprehensive dataset on axillary radiological features. In addition, the majority of our patients with positive needle biopsy or SLNB results had ALND; hence, the true axillary nodal status was accurately reflected. Our ultrasound sensitivity and specificity rate were also comparable with those reported in previous studies examining patients with early breast cancer [
3171819].
Our study had some limitations. Firstly, as this was a retrospective study, the number of abnormal axillary LNs may not have been purposefully sought for at the time of the ultrasound examination. Secondly, the patients with N0 did not undergo an ALND to verify the accuracy of their axillary nodal status. However, an average of 2.83 LNs were harvested from these patients, which was very similar to the 3 LNs reported in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial in which the performance of SLNB was standardized [
20]. In the Z0011 trial on SLNB arm, the median number of sentinel LNs obtained was 2, which again was comparable to that reported in our study.
In conclusion, 4 or more abnormal LNs detected on ultrasound was highly predictive of HNB in the early breast cancer patient cohort. Detection of these abnormal LNs allows the preoperative identification of patients who require upfront ALND and thus avoid SLNB.
ACKNOWLEDGMENTS
We would like to acknowledge Dr Hannah Angela Acosta and Dr Jayne Michelley Adolfo Lim who helped with part of the data collection.
References
3. Bailey A, Layne G, Shahan C, Zhang J, Wen S, Radis S, et al. Comparison between ultrasound and pathologic status of axillary lymph nodes in clinically node-negative breast cancer patients. Am Surg. 2015; 81:865–869.


5. Synnestvedt M, Borgen E, Russnes HG, Kumar NT, Schlichting E, Giercksky KE, et al. Combined analysis of vascular invasion, grade, HER2 and Ki67 expression identifies early breast cancer patients with questionable benefit of systemic adjuvant therapy. Acta Oncol. 2013; 52:91–101.


6. Dengel LT, Van Zee KJ, King TA, Stempel M, Cody HS, El-Tamer M, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014; 21:22–27.


7. Sharifi S, Peterson MK, Baum JK, Raza S, Schnitt SJ. Assessment of pathologic prognostic factors in breast core needle biopsies. Mod Pathol. 1999; 12:941–945.

8. Pilewskie M, Mautner SK, Stempel M, Eaton A, Morrow M. Does a positive axillary lymph node needle biopsy result predict the need for an axillary lymph node dissection in clinically node-negative breast cancer patients in the ACOSOG Z0011 era? Ann Surg Oncol. 2016; 23:1123–1128.


9. Humphrey KL, Saksena MA, Freer PE, Smith BL, Rafferty EA. To do or not to do: axillary nodal evaluation after ACOSOG Z0011 trial. Radiographics. 2014; 34:1807–1816.


10. Boland MR, Prichard RS, Daskalova I, Lowery AJ, Evoy D, Geraghty J, et al. Axillary nodal burden in primary breast cancer patients with positive pre-operative ultrasound guided fine needle aspiration cytology: management in the era of ACOSOG Z011. Eur J Surg Oncol. 2015; 41:559–565.


11. Lim GH, Upadhyaya VS, Acosta HA, Lim JM, Allen JC Jr, Leong LC. Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result. Eur J Surg Oncol. 2018; 44:945–950.


12. Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast. 2012; 21:678–681.


14. Wang L, Yu JM, Wang YS, Zuo WS, Gao Y, Fan J, et al. Preoperative lymphoscintigraphy predicts the successful identification but is not necessary in sentinel lymph nodes biopsy in breast cancer. Ann Surg Oncol. 2007; 14:2215–2220.


15. Bézu C, Coutant C, Salengro A, Daraï E, Rouzier R, Uzan S. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol. 2011; 20:e55–9.

16. Bishop JA, Sun J, Ajkay N, Sanders MA. Decline in frozen section diagnosis for axillary sentinel lymph nodes as a result of the American College of Surgeons Oncology Group Z0011 trial. Arch Pathol Lab Med. 2016; 140:830–835.


17. del Riego J, Diaz-Ruiz MJ, Teixidó M, Ribé J, Vilagran M, Canales L, et al. The impact of preoperative axillary ultrasonography in T1 breast tumours. Eur Radiol. 2016; 26:1073–1081.


18. Cools-Lartigue J, Sinclair A, Trabulsi N, Meguerditchian A, Mesurolle B, Fuhrer R, et al. Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: predictors of accuracy and future implications. Ann Surg Oncol. 2013; 20:819–827.


19. Farshid G, Kollias J, Grantley Gill P. The clinical utility of assessment of the axilla in women with suspicious screen detected breast lesions in the post Z0011 era. Breast Cancer Res Treat. 2015; 151:347–355.


20. Olaya W, Wong J, Wong J, Morgan J, Kazanjian K, Lum S. When is a lymph node dissection a lymph node dissection? The number of lymph nodes resected in sentinel and axillary lymph node dissections. Ann Surg Oncol. 2013; 20:627–632.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download