This article has been
cited by other articles in ScienceCentral.
Abstract
The luteinizing hormone-releasing hormone/androgen receptor (LHRH/AR) pathway is a promising treatment target in a subgroup of female patients with triple-negative breast cancer (TNBC). However, very little is known about the efficacy of this strategy in male patients with TNBC. In this report, we describe a male patient with AR-positive TNBC who was successfully treated using an LHRH agonist after pretreatment with several lines of chemotherapy and achieved a durable response. We also review the existing evidence supporting LHRH- and AR-targeted therapy for this rare disease.
Keywords: Luteinizing hormone-releasing hormone, Androgen receptor, Triple-negative breast neoplasms
INTRODUCTION
Approximately 15% of all breast cancers lack the expression of the estrogen receptor(ER), progesterone receptor(PR), and human epidermal growth factor receptor 2 (HER 2). Hence, these lesions are classified as triple-negative breast cancer (TNBC). Evidence clearly indicates that the lack of effective targeted therapies that can prolong unfavorable survival patients with TNBC is the major challenge associated with the management of these patients. Data from several recent genomic studies have indicated that TNBC comprises a group of diverse cancers with unique molecular abnormalities that may serve as novel treatment targets [
1]. Although these studies did not define identical TNBC subtypes, 1 consistent finding was the presence of a luminal androgen receptor (LAR) subtype in 10%–15% of patients [
1]. These LAR cells display luminal gene expression and a complete absence of basal-like markers prevalent in the majority of TNBCs. Importantly, LAR cells are characteristically enriched androgen receptor (AR) gene targets, and their growth is dependent—at least in part—on AR signaling [
2]. In pre-clinical studies, the knockdown or anti-androgen-mediated pharmacological inhibition of AR was shown to greatly decrease cell viability and tumor growth [
2]. These findings strongly suggest a potential role of anti-androgens in the treatment of this peculiar TNBC subtype. Subsequently, early reports from many phase II clinical trials have reported encouraging efficacy outcomes of these agents in females with AR-positive metastatic TNBC (mTNBC).
In this report, we describe a male patient with AR-positive mTNBC who was successfully treated with a luteinizing hormone-releasing hormone (LHRH) agonist after pretreatment with several lines of chemotherapy.
CASE REPORT
A 48-year-old male first presented to our hospital in November 2008 with a right axillary mass (4 cm) and no clinically or radiologically detected breast lesions. A Tru-Cut needle biopsy revealed a grade III mammary carcinoma, and immunohistochemistry (IHC) confirmed a TNBC phenotype. The tumor cells tested strongly positive for cytokeratin 7 (clone OV-TL 12/30, FLEX monoclonal mouse anti-human, DAKO; Agilent Technologies Korea Ltd., Seoul, Korea), carcinoembryonic antigen (clone II-7, FLEX monoclonal mouse anti-human, DAKO Omnis; Agilent Technologies Korea Ltd.), and GATA binding protein 3 (GATA 3) (L50-823, mouse monoclonal primary antibody; CELL MARQUE Corp., Rocklin, USA); negative for both the ER (SP1, rabbit monoclonal primary antibody; Ventana Medical Systems, Tucson, USA) and PR (1E2, rabbit monoclonal primary antibody, VentanaMedical Systems); and negative (score: 1+) for HER 2 overexpression (4B5, rabbit monoclonal primary antibody, Benchmark XT;Ventana Medical Systems). A metastatic workup did not reveal any disease outside the axilla, and the concentrations of serum tumor markers, including carcinoembryonic antigen, α-fetoprotein, cancer antigen (CA)19-9, CA 125, CA15-3, and prostate-specific antigen, were all within normal levels. The patient underwent complete axillary lymph node dissection, which revealed the involvement of all 15 dissected lymph nodes and extra-nodal soft tissue invasion. The patient was staged as Tx/N3/M0 and scheduled to receive standard adjuvant chemotherapy and postoperative locoregional radiotherapy. However, the patient refused this plan and was placed under follow-up.
From November 2009 to October 2014, the patient experienced 5 events of locoregional recurrence but exhibited no evidence of distant metastases. During this period, disease management comprised a right mastectomy and locoregional irradiation, as well as repeated limited surgical resection of the recurrent right chest wall nodules. The patient was offered 2 pseudo-adjuvant chemotherapy regimens following his first and second recurrence events: the first was the FEC100 regimen, comprising 5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks, and the second was capecitabine 1,750 mg/m2 on days 1–14. He refused to receive any further chemotherapy for subsequent events. The results of histopathologic and IHC examinations of all surgically excised tissues were concordant with a TNBC phenotype.
In October 2015, the patient developed minimally symptomatic bone metastases that exhibited active tracer uptake on positron emission tomography/computed tomography (PET/CT). He was treated with weekly paclitaxel (75 mg/m
2/week as a 1-hour infusion), which was well tolerated; after 16 weeks of this regimen, he achieved complete pain alleviation and a partial regression of his bone lesions, as evidenced by a PET/CT scan. The patient completed a total of 24 weeks of chemotherapy, which was stopped due to evolving grade II sensory neuropathy. Three months later, he began to exhibit clinical deterioration as indicated by a low-grade fever, progressive generalized bone pain, and fatigue. At this time, a PET/CT scan revealed an increase in the number and activity of bone lesions, as well as multiple skin nodules overlaying the right chest wall. The indolent nature of the disease led us to suspect a LAR subtype. A new skin lesion biopsy revealed a TNBC phenotype with a Ki-67 positivity rate of 17%. According to IHC, the tumor cells were strongly positive for AR (100% of malignant cells) and GATA 3 (
Figure 1). Additionally, his serum CA 15-3 level increased by more than 12 times the normal value.
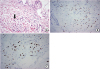 | Figure 1
Microscopic findings. (A) H&E staining indicates an invasive ductal carcinoma. Metastatic skin nodule showing dermal infiltration by cancer cells with eccentric hyperchromatic nuclei (arrow) (×400). (B) IHC reveals GATA 3 positivity and AR positivity. Tumor cells show positive GATA 3 nuclear stain with moderate to strong intensity (×400). (C) One hundred percent of tumor cells are strongly positive for AR (×400).
H&E = hematoxylin and eosin; IHC = immunohistochemistry; AR = androgen receptor; GATA 3 = GATA binding protein 3.

|
Given the encouraging early results from studies of anti-androgens in females with AR-positive mTNBC, we decided to apply the same approach to the management of our patient. Accordingly, we discussed this treatment option with the patient who consented to receive this therapy. The first dose of LHRH agonist (goserelin 3.6 mg subcutaneously every 28 days) was administered in August 2016, and the patient was advised to initiate bicalutamide at 150 mg/day (enzalutamide was not available). The patient, however, did not use this oral anti-androgen agent because of fears that it would exacerbate his anticipated sexual dysfunction. During his initial clinical assessment conducted 3 weeks after the first goserelin injection, the patient reported complete symptomatic relief and achieved an almost complete resolution of his recurrent chest wall nodules. This very remarkable early response encouraged our decision to continue goserelin monotherapy. Three months later (November 2016), the patient remained symptom-free, while his PET/CT scan revealed a partial response (
Figure 2). After 6 months on therapy, the patient remained symptom-free, and his CA 15-3 level decreased to 19.4 U/mL (compared to 409.5 U/mL at the start of treatment) (
Figure 3). However, he reported a marked intolerance to goserelin, as indicated by grade III hot flushes and sexual dysfunction, and refused to undergo further treatment. On May 22, 2017, the patient again presented with a low-grade fever and rapidly progressive bone pain. A new PET/CT assessment revealed progression in the number and activity of the metastatic bone lesions, which was accompanied by a markedly elevated serum CA 15-3 level (
Figures 3). Accordingly, the patient restarted goserelin and was instructed to add bicalutamide (150 mg per os daily). After another refusal to receive bicalutamide for fear of aggravating the goserelin-related adverse events, he decided to continue goserelin monotherapy. The patient again experienced extremely rapid symptomatic relief within the first 2 weeks of goserelin re-initiation. A new PET/CT scan after 6 months revealed a complete remission of all metabolically active lesions and a dramatic decrease in the serum CA15-3 level to 19.7 U/mL. He remained on goserelin monotherapy until the last injection on July 5, 2018, with no disease-related symptoms other than persistent grade II hot flushes and sexual dysfunction.
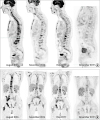 | Figure 2
Serial PET/CT scans at the initiation of anti-androgen therapy and during the subsequent year. (A) Sagittal (B) Coronal sections.
PET/CT = positron emission tomography/computed tomography.

|
 | Figure 3
Graphical plotting of serial CA 15-3 measurements at the initiation of anti-androgen therapy and during the subsequent year.
CA = cancer antigen.

|
DISCUSSION
Male breast cancer is a rare disease, accounting for less than 1% of all breast cancers. Notably, the vast majority of male breast cancers are ER-positive tumors, whereas TNBCs are rarely seen, although the underlying reasons are poorly understood. In the first report of the International Male Breast Cancer Program, which entailed a central pathology review of tumor samples from 1,483 males with breast cancer, less than 1% of the samples were classified as the TNBC subtype [
3].Accordingly, systematic studies of the natural histories and treatment outcomes of males with TNBC are practically inexistent, and not surprisingly, no published report has discussed the LAR subtype among males.
In females, the natural history of LAR TNBC was recently characterized as a rather indolent disease that responds modestly to chemotherapy but has an apparently better prognosis relative to other TNBC subtypes. Notably, LAR tumors predominantly tend to spread to the lymph nodes, soft tissues, and bones, whereas basal-like TNBCs are known to preferentially metastasize to visceral organs, especially the lungs and brain. In clinical trials, AR protein expression, determined by IHC, was used as a robust surrogate for the LAR genomic subtype. The frequency of AR-positive TNBC has been reported to range from ≤ 10% to ≥ 50%. Intriguingly, AR expression is not exclusively observed in the LAR molecular subtype and can be detected at lesser intensities in other TNBC subtypes [
4]. We strongly believe that our case is analogous to the LAR-subtype described in females, given the patient's unique clinical features and the presence of IHC markers suggestive of luminal disease in his tumor samples (GATA 3 and gross cystic disease fluid protein 15 positivity and strong AR expression in 100% of tumor cells).
The AR-signaling pathway was recently identified as a unique therapeutic target in patients with LAR tumors, who would otherwise be treated via chemotherapy. Gucalp et al. [
5] reported the first clinical trial on the use of bicalutamide (150 mg daily) in 26 patients with AR-positive TNBC (defined as > 10% AR nuclear staining by IHC). In that trial, the 24-week clinical benefit rate (CBR24) was 19%, and the median progression-free survival (mPFS) duration was 12 weeks. Although some patients in this trial exhibited volumetric tumor regression, none met the Response Evaluation Criteria In Solid Tumors criteria for a partial response. Nevertheless, a subsequent case report reported a complete remission in a females with AR-positive mTNBC who received the same treatment[
6]. A subsequent larger trial reported the use of the more potent AR antagonist enzalutamide (160 mg/day). In this trial, patients with a AR positivity rate > 10% (75 cases) had a CBR of 33% at 16 weeks, and 5 achieved confirmed objective responses as a result of enzalutamide therapy [
7]. Importantly, in a predefined analysis that used a novel genomic assay to quantitate AR-related RNA expression (dividing patients into diagnostic test positive [Dx+] and negative [DX−] population), patients with Dx+ TNBC (nearly half of the intention-to-treat population) had more favorable outcomes than those with Dx− tumors in terms of the clinical benefit rate at 24 weeks (CBR24) (36% vs. 6%) and mPFS duration (16.1 weeks vs. 8.1 weeks) [
8]. Notably, Dx+ patients who received enzalutamide as a first- or second-line therapy had a mPFS duration of 32 weeks, comparable to the duration achieved using a classic endocrine therapy among females with ER-positive disease. These results certainly highlight the importance of appropriate patient selection based on tumor genomics to achieve the best treatment outcome of such a novel approach. The prospective validation of this diagnostic assay may identify as many as 50% of patients with AR-positive TNBC who might benefit from enzalutamide.
Some small retrospective studies and a few case studies have reported the use of anti-androgens, either alone or in combination with LHRH agonists, for male breast cancer [
9,
10]; however, their use had not been previously reported in males with a TNBC phenotype (
Table 1). In the largest case series, Di Lauro et al. [
10] treated 36 male patients with metastatic breast cancer using cyproterone acetate either with or without a LHRH analog during the period of 1975–1999. Of the 7 cases with a known AR status, 1 had AR-positive/ER-negative (but PR+) disease and achieved a complete response with a combination of cyproterone acetate and goserelin [
10].
Table 1
Clinical cases/studies that used LHRH agonists in male breast cancer

|
Author |
Disease stage/biology |
Androgen targeting drugs |
Sample size |
Response rate |
|
Labrie et al., 1990 [9] |
Case of met. MaBC |
Flutamide + LHRH agonist |
1 |
Radiologic CR |
|
Di Lauro et al., 2014 [10] |
Expanded case series, Met. MaBC, 3 AR+/ER+ cases and 1 AR+/ER− |
CPA alone |
14 |
12 |
|
CPA + LHRH |
22 |
18 |
|
Current case |
Met. AR+/ER− MaBC |
LHRH agonist |
1 |
Radiologic PR |

To the best of our knowledge, ours is the first case report of a male patient with a confirmed diagnosis of AR-positive mTNBC who was treated via endocrine therapy. Although we originally planned to treat this patient with combination of an LHRH agonist and the oral anti-androgen bicalutamide, the patient refused the latter drug. Notably, this patient experienced an exceptionally early clinical improvement in response to LHRH agonist monotherapy, and a similar response was observed upon retreatment with the same agent. This strikingly consistent response was apparently associated with the presence of a dominantly AR-driven tumor, which responded promptly to androgen suppression induced by the LHRH agonist. However, the observed rapid response may also have been related to the potential therapeutic effects of targeting LHRH receptor (LHRH-R) expression in TNBCs. LHRH-R is known to be expressed by many human tumors, including breast cancer. For example, approximately 50% of patients with TNBC exhibited LHRH-R expression, although the impact on prognosis has not yet been reported.
In vitro studies have shown that treatment with LHRH analogs was associated with growth inhibition and apoptotic cell death in the LHRH-R-positive TNBC cell lines MDA-MB-231 and HCC1806, with synergistic anti-cancer effects when coadministered with chemotherapy [
11].
The particular interest in this paradigm resulted from the POEMS study, which tested the role of goserelin in fertility preservation in young patients with breast cancer [
12]. That study randomly assigned 257 premenopausal females with ER-negative breast cancer (85% with a TNBC phenotype) to receive standard chemotherapy plus goserelin or chemotherapy alone. Notably, the study reported improved rates of disease-free survival (adjusted hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.24–0.97;
p = 0.04) and overall survival [OS] (adjusted HR, 0.43; 95% CI, 0.18–1.00;
p = 0.05) in the goserelin group. A subsequent retrospective Korean study suggested the same conclusion, and a more recent meta-analysis of 4 trials suggested that the use of gonadotropin-releasing hormone analogs to preserve fertility was associated with a trend toward improved OS in females with early-stage TNBC [
13,
14].
In conclusion, we reported the natural history of a male patient with a LAR-like mTNBC who responded markedly and successfully to LHRH agonist treatment. The treatment decision was extrapolated from recently published data on the potential benefit of AR-targeted treatment in females with the same disease subtype. Clinical treatment strategies for male breast cancer are often based on recommendations for different subtypes of female breast cancer. Despite many molecular similarities between female and male breast cancers, growing evidence suggests that male breast cancer is not genomically concordant with the well-established intrinsic subtypes of female breast cancer. Therefore, prospective trials dedicated to male breast cancers are urgently needed. Given the rarity of the disease, international collaborations would certainly be needed to increase the sample sizes for clinical studies and translational research.






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download