INTRODUCTION
Improvement in the technique of auxiliary examination, advanced diagnosis, and a better awareness of self-examination have resulted in an increasing number of women diagnosed with bilateral breast cancer (BBC) [
12]. The incidence rate of BBC in the literature ranges from 4% to 7% [
34]. When a patient develops BBC, whether it is metastasis that spread from one side of the breast to the other or a second primary event that developed in the other breast, correct diagnosis is essential not only for treatment, but also for understanding the prognosis [
56]. However, there have not been any standard identification criteria to date.
Over the past few decades, great strides have been made in cancer genomics, both in the clinic and in the research of malignant diseases. Exploration of genomic sequences and molecular data have revealed fundamental alterations in the structure or copy number of genes, providing evidence at an undetectable, genetic level [
78]. Mutational profiling data of 2 tumors, in a wide range of cancers, have been investigated and used to identify their clonal relationship [
910].
Here, we report a rare case of synchronous BBC in a 61-year-old Chinese woman, where we aimed for a correct and convincing diagnosis through the utilization of whole exome sequencing (WES).
CASE REPORT
In April 2017, a 61-year-old Chinese woman, presenting with a palpable lump in her right breast, was admitted to our department. On physical examination, a conspicuous lump was noted in the upper inner quadrant of the right breast, with a dimple in the overlying skin. There was no palpable mass in the left breast. An ultrasound scan showed a 4.4 × 2.4 cm irregular low echo in the upper inner side of the right breast, and a 1.2 × 0.6 cm irregular low echo was found in the nipple areola area of the left breast. Enlarged lymph nodes (LNs) were observed in both axilla (
Figure 1A and B). Magnetic resonance imaging (
Figure 1C and D) and a mammogram (
Figure 1E-H) further confirmed the bilateral masses and respectively enlarged axillary LNs.
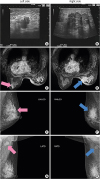 | Figure 1
Imaging studies. (A) The 3 o'clock view of the areola area of the left breast; 1.0 cm away from the body surface, a hypoechoic mass of 1.2 × 0.6 cm with an irregular shape and unclear boundary can be seen. (B) The 12–3 o'clock view of the right breast; 0.7 cm away from the body surface, a hypoechoic mass of 4.4 × 2.4 cm with a “crab-feet” change and clear margin can be seen. (C) An abnormal signal is visible at the rear of the left nipple, about 1.7 × 0.9 cm in size. (D) The right breast has a large, abnormal signal mass, about 4.4 × 3.3 cm in size and lobulated, which is mainly distributed within the upper quadrant, and its edge has burrs. There is right nipple retraction, and the inner, upper quadrant of the skin is thickened. Mammograms of the mediolateral oblique (E, F) and AT (G, H) positions. Mammograms of the AT position show a hint of bilateral, lymphatic metastasis.
AT = axillary tail.

|
We further carried out core needle biopsy of both breast masses and the enlarged axillary LNs. The mass in the left breast was pathologically diagnosed as an invasive ductal carcinoma (IDC), grade II, estrogen receptor (ER) 3+ (95%), progesterone receptor (PR) 1+ (< 1%), human epidermal growth factor receptor 2 (HER2) 1+, and Ki-67 30% (
Figure 2A-E). The mass in the right breast was an IDC with neuroendocrine characteristics, grade II, ER 2+ (50%), PR−, HER2−, and Ki-67 30% (
Figure 2F-J). Metastatic cancer lesions were found in the axillary LNs bilaterally (
Figure 3).
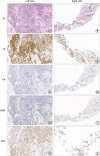 | Figure 2
Analysis of the biopsy tissues from the bilateral breast tumors. (A) The section on the left tumor showed an IDC, grade II (H & E × 250). (B-E) The left tumor revealed ER (95% 3+), PR (< 1% +), HER2 (1+), and Ki67 (30%) (IHC × 250). (F) The section on the right tumor showed an IDC, grade II (H & E × 250). (G-J) The right tumor revealed ER (50% 2+), PR (−), HER2 (−), Ki67 (30%) (IHC × 250).
H & E = hematoxylin and eosin; IDC = invasive ductal carcinoma; IHC = immunohistochemistry; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.

|
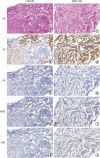 | Figure 3
Analysis of the biopsy tissues of the bilateral axillary LNs. (A) The LN of left axilla showed an IDC, grade II (H & E,× 250) (B-E) The LN of the left axilla revealed ER (60% 3+), PR (−), HER2 (1+), and Ki67 (30%) (IHC, × 250). (F) The LN of the right side showed an IDC, grade II (H & E, × 250). (G-J) The LN of the right axilla revealed ER (90% 3+), PR (< 1% +), HER2 (−), and Ki67 (25%) (IHC, × 250).
LN = lymph node; H & E = hematoxylin and eosin; IHC = immunohistochemistry; IDC = invasive ductal carcinoma; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.

|
Further computed tomography scans of the chest and upper abdomen and a bone scan showed no evidence of metastatic disease. All of the clinical and histopathological characteristics of the patient's BBC are shown in
Table 1.
Table 1
Clinical and pathological characteristics of BBCs and axillary LNs

|
Variable |
Left |
Right |
|
Site of the lump |
Upper outer quadrant |
Upper inner quadrant |
|
Size |
1.7 × 0.9 cm |
4.4 × 3.3 cm |
|
Skin involvement |
No |
Yes |
|
Pathology (biopsy, pre-treatment) |
IDC, grade II |
IDC, grade II |
|
ER 95% 3+, PR < 1%+, HER2 1+, Ki-67 30% |
ER 50% 2+, PR−, HER2−, Ki-67 30% |
|
Axillary LNs |
Luminal B HER2− |
Luminal B HER2− |
|
IDC, grade II |
IDC, grade II |
|
ER 60% 3+, PR−, HER2 1+, Ki-67 30% |
ER 90% 3+, PR < 1%+, HER2−, Ki-67 25% |
|
Luminal B HER2− |
Luminal B HER2− |
|
Pathology (post operation) |
IDC, grade II multifocal surrounded by DCIS peritumoral intravascular cancer emboli |
IDC, grade II |
|
Lymphatic metastasis |
pN2 (7/14) |
pN2 (8/10) |

Therefore, this patient was diagnosed with BBC. Right breast: IDC, grade II, cT4 (skin involved) N1M0, luminal B HER2 negative; and left breast: IDC, grade II, cT1c (1.7 × 0.9 cm) N1M0, luminal B HER2 negative. She received 6 cycles of neoadjuvant chemotherapy comprising capecitabine (1,500 mg orally twice daily on days 1–14, cycled every 21 days) plus docetaxel (75 mg/m2 intravenously on day 1, cycled every 21 days) and underwent bilateral modified radical mastectomy in October 2017. The postoperative pathological diagnoses for the right breast were IDC, grade II, and the tumor diameter was 3.0 cm; for the left breast, multifocal IDC, grade II, and a tumor diameter of 1.0 cm, peritumoral intravascular cancer emboli, and a low-grade ductal carcinoma in situ (DCIS) could be observed. Surgical staging was ypT2 (3 cm) N2 (8/10) for the right breast and ypT1b (1 cm) N2 (7/14) for the left breast.
Confusingly, when it came to the next treatment, we needed to verify whether her contralateral breast cancer was a metastatic lesion or a second primary event. However, based solely on her clinical and pathological features, we could not positively identify its origin. Therefore, in order to know her cancer genetic information, we utilized WES and cancer genome analysis for each tumor sample to assist our discrimination.
From the somatic mutation heatmap, we observed that, besides unique variations, there were 34 common mutations between the left and right breast lesions (
Figure 4A). The variation frequency (VAF) distribution showed that these mutations mainly belonged to the founder mutation, which originated from the only tumor clone, and the correlation coefficient was 0.77 (
Figure 4B).
 | Figure 4
Analysis of whole exome sequencing research. (A) Mutation heatmap for the BBCs and axillary LNs. All L, LN, R samples share 9 common variations which are not found in RN. These variations are AK3, PIEZO1, OR8B3, WDR17, PLXNA1, PCYT1A, ZNF629, TRDMT1 and OR6B3. (B) VAF distribution (%) for the BBCs and axillary LNs. The mean VAF of those shared common variations are 5%, 15% and 16% in L, LN and R samples respectively.
Yellow = a gene with a mutation; Blue = a gene without mutation; LN = lymph node; BBC = bilateral breast cancer; VAF = variation frequency.

|
Further, the whole genome copy number variations (CNVs) in the left breast tumor were similar to those in the right lesion. Chr8 and chr11 displayed a high gene-amplification pattern, such as the interval
CCND1,
FGF3, and
FGF4 in chr11 (
Figure 5). More importantly, compared to the left breast cancer, we noticed a loss of heterozygosity (LOH) of chr6/11/13 in the right breast cancer (
Figure 6).
 | Figure 5
CNVs of the BBCs. CNVs in the left breast tumor were similar to those in the right lesion. Chr8 and chr11 displayed a high gene-amplification pattern.
CNV = copy number variation; BBC = bilateral breast cancer.

|
 | Figure 6
The CNV distribution of all chromosomes. Chr6/11/13 LOH were shown in the right breast cancer.
CNV = copy number variation; LOH = loss of heterozygosity.

|
Additionally, we sequenced the axillary metastatic LNs bilaterally (
Figure 4). As demonstrated in
Figure 4A, the same yellow area, which means a majority of the same genes with mutations, could be found among the left breast cancer, left axillary metastatic LNs, and right breast cancer, but not in the right axillary metastatic LNs. Moreover, the VAF distribution revealed differences in the LNs on the right side, which has been illustrated in intervals, such as
AK3,
OR8B3,
PCYT1A, and
OR6B3.
The patient's next treatment course was based on the traditional regimen for metastatic breast cancer. She received postoperative irradiation and capecitabine (1,500 mg orally twice daily on days 1–14, cycled every 21 days) plus letrozole (2.5 mg orally once per day). She is on a regular follow-up every 3 months. Thus far, it has been 1 year since her operation. No recurrence or metastasis has been detected by routine imaging.
DISCUSSION
In 1964, based on clinical and histopathological parameters, Robbins and Berg [
11] defined guidelines for a second primary lesion as follows: first, different histological types of tumors; second, lesions of DCIS are present in both breasts; third, the same histological types in the tumors in both breasts, but with no evidence of local, regional, or systemic metastases; and fourth, the predilection site of the primary cancer is the upper outer quadrant of the breast, and it always appears alone. However, the majority of breast cancers share the same pathological feature, which is an IDC. Due to the complications of the clinical signs and tumor heterogeneity, these criteria could not be satisfied in the distinction between the second primary site and the true recurrence of BBC [
1213]. Therefore, in this case, according to the clinical and histopathological guidelines mentioned above [
11] (
Table 2), we could only say that she was inclined to, but did not definitely, have metastatic breast cancer from 1 breast to the other.
Table 2
Patient summary compared to the guidelines described by Robbins and Berg [11] in 1964

|
Variable |
Left |
Right |
Comparing to the guidelines |
|
Pathology |
IDC, grade II |
IDC, grade II |
Non-conformity |
|
Present of DCIS |
YES |
NO |
Non-conformity |
|
Lymphatic metastasis |
YES |
YES |
Non-conformity |
|
Site of the lump |
3 o'clock of areola area |
Upper inner quadrant |
Non-conformity |

Based on molecular characteristics, Banelli et al. [
14] evaluated the distinct origins of 2 tumors through different X-inactivation analyses, but this method is uninformative if both have the same X-chromosome status. Janschek et al. [
15] posited that if mutations in the
P53 gene in 2 tumors are the same, it might be a marker for identical progenitor cells. However, there might not be strong evidence of this phenomenon, because mutations of
P53 are common, and germline mutations are also possible. Limited information could be grasped from such gene segments, which resulted in unconvincing statistical power for correct classification. A more feasible and cost-effective approach should be presented for an auxiliary diagnosis.
In this case, we utilized WES in the sequencing of paraffin-embedded specimens from the breast tumors on both sides. The results inferred a conclusion of metastasis. The somatic mutation heatmap from the bilateral lesions revealed that 34 of the mutations were the same, and these mainly belonged to the founder mutation and originated from 1 tumor clone according to the VAF (
Figure 4). Additionally, similar CNVs were seen in both breasts. Together, this revealed that the 2 lesions had the same origin and that there might be an evolution from one side to the other.
The primary lesion and metastatic lesion needed to be identified. At first glance, the right breast tumor might be considered as the primary lesion, because the right lump was bigger. However, after a careful review according to the guidelines mentioned above, we finally came to the opposite conclusion. A DCIS was detected in the left breast (
Table 1). The lump on the left was located in the upper outer quadrant, while the right tumor was in the upper inner quadrant of the breast. Additionally, from the genetic profiling data, more mutations were found in the right breast tumor and a LOH was detected in chr6/11/13 of the right breast tumor, which indicates a genetic evolution from left to right breast cancer. Moreover, additional sequencing of the bilateral axillary metastatic LNs also strengthened this result. We found a common clonal population in the left breast lesion, left axillary metastatic LNs, and right breast lesion; but not in the right axillary metastatic LN. This suggests the evolution from left to right, and the right axillary metastatic LNs were the last lesions to develop.
Conclusively, the clinical, histopathological and genomic data revealed that the patient developed contralateral metastatic breast cancer, which is a metastasis from the left to the right breast lesion. Based on personal clinicopathological data, WES might be a comprehensive and convincing supplementary technique for making an accurate differential diagnosis for BBC.












 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download