Human granulocytic anaplasmosis (HGA) caused by the rickettsial bacterium
Anaplasma phagocytophilum is transmitted by
Ixodid ticks that infect mammals including humans.
12 Since the identification of HGA in the United States in 1994, the number of HGA cases has increased steadily from 348 cases in 2000, to 4,151 cases in 2016.
3 In comparison to the United States, Europe shows a much lower incidence of HGA. It is increasingly described in eastern parts of Asia, especially China, Japan, and Korea.
1
Many previous studies based in Korea have focused on anaplasmosis in animals other than humans,
45 indicating a long-term interest in the disease. Since the first HGA case was reported in 2013,
2 few cases have been reported in Korea.
6
Scrub typhus, hemorrhagic fever with renal syndrome (HFRS), leptospirosis, and severe fever with thrombocytopenia syndrome are common infectious diseases reported in Korea, especially in autumn. HGA is still considered a rare infectious disease in Korea. Recently, we have encountered some cases in which patients have co-infection of HGA and scrub typhus or HFRS, and we have also observed seasonal variation in HGA cases in Korea.
All adult patients aged 18 years of age or older who were clinically suspected and laboratory-confirmed to be infected with A. phagocytophilum were enrolled between January 2015 and September 2018 at two tertiary university hospitals in Korea: Chonbuk National University Hospital (1,200 beds) and Wonkwang University Hospital (760 beds). Demographic data, clinical manifestations, and results of laboratory tests were collected in a retrospective review of electronic medical records.
Indirect immunofluorescent assay (IFA) tests to detect immunoglobulin (Ig) G, IgM, and IgA antibodies against the standard
Orientia tsutsugamushi antigens from the Gilliam, Karp, and Boryong strains (Green Cross Reference Laboratory, Yongin, Korea) were performed. Scrub typhus was diagnosed by a single titer ≥ 1:160 or a ≥ 4-fold rise in IFA titer in paired serum samples.
7 HGA was diagnosed by a ≥ 4-fold rise in IFA (IgG or IgM) titer (Fuller Laboratories, Fullerton, CA, USA) in paired serum samples or positive polymerase chain reaction (PCR) test using 16S ribosomal RNA (rRNA) as a target gene of
A. phagocytophilum in patient blood samples. Extracted DNA from blood was used to amplify fragments of the 16S rRNA genes of
A. phagocytophilum. 16S PCR primers for
Anaplasma and
Ehrlichia species were AE1_F (5′-AAGCTTAACACATGCAAGTCGAA-3′) and AE1_R (5′-AGTCACTGACCCAACCTTAAATG-3′), and nested PCR primers for
A. phagocytophilum were AP_F (5′-GTCGAACGGATTATTCTTTATAGCTTGC-3′) and AP_R (5′-CCCTTCCGTTAAGAAGGATCTAATCTCC-3′).
8 HFRS was diagnosed by a ≥ 4-fold rise in IFA titer (Green Cross Reference Laboratory) for detection of
Hantaan virus in paired serum samples.
This study was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. The study was approved by the institutional review board (IRB) of Chonbuk National University Hospital, and all patients provided written informed consent (IRB registration number 2015-10-007).
A total of 17 patients who fulfilled the diagnostic criteria of HGA were identified. Demographic and clinical characteristics of the patients are summarized in
Table 1. The mean age was 74.4 years. There were 7 men and 10 women. Among 17 cases, the mean time from symptom onset to admission was 4.1 days. Fever was found in all patients, and skin rash and gastrointestinal symptoms were common. Although 1 patient received intensive care, all patients with HGA survived with good clinical outcomes.
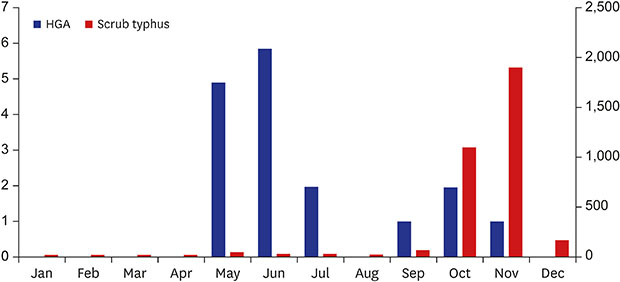
Different seasonal variations have been reported in HGA and scrub typhus. Scrub typhus occurs most often in October and November in Korea,
910 and a peak in HGA cases typically occurs earlier, in June and July.
3 Our study, however, found that monthly incidence peaked in May and June (
Fig. 1).
Among 17 patients with HGA, 3 had both HGA and scrub typhus, and 1 had both HGA and HFRS. Among 3 patients who had co-infection of HGA and scrub typhus, 2 occurred in October and November, and 1 occurred in May. The patient who had co-infection of HGA and HFRS occurred in June. Co-infection was diagnosed if the serologic results of the IFA test fulfilled the diagnostic criteria for both infections. Three patients with scrub typhus were diagnosed by a single titer ≥ 1:160, and 1 patient with HFRS was diagnosed by a ≥ 4-fold rise in IFA titer in paired serum samples (
Table 2). In addition, co-infected patients had not only serologic results but also clinical manifestations relevant to scrub typhus or HFRS. The patient with HFRS recovered after presenting with proteinuria, thrombocytopenia and renal impairment. But we could not find any significant differences observed in clinical symptoms between HGA patients and co-infected patients.
In conclusion, the occurrence of HGA in Korea has been steadily rising. In 2002, 1.8% of serum samples from Korean patients with acute febrile diseases were positive for the
A. phagocytophilum antibody by indirect immunofluorescence assay.
11 The number of patients who suspect they have the infection has more than doubled from 201 (6.96% seroreactivity of IFA test and 2.54% positivity of PCR test) in 2015 to 598 (9.36% seroreactivity of IFA test and 8.38% positivity of PCR test) in 2017 in Korea.
12 In 2003, molecular epidemiologic study detected
A. phagocytophilum in
Haemaphysalis longicornis and
Ixodes persulcatus ticks from Korea at a rate of 9.9%.
13 In 2011, using 16S rRNA-based nested PCR,
A. phagocytophilum was detected in 63.6% of Korean water deer.
4 Following this, the first patient with HGA was reported in Korea in 2013.
2 Since then, the number of suspected patients with HGA has increased steadily.
Scrub typhus, HFRS, and leptospirosis are common zoonotic diseases in Korea, with high prevalence in rural areas during autumn. A sharp peak in the number of scrub typhus cases occurs during October and November in Korea.
9 Similarly, HFRS and leptospirosis predominantly occur during the last quarter of the calendar year.
1415 In contrast, the majority of HGA cases have illness onset during the summer months.
3 Our study reports an earlier peak of HGA in May and June. The seasonal variation pattern of these diseases can be helpful in differential diagnosis.
Co-infection of HGA and Lyme disease or babesiosis has been reported because they share the deer tick,
I. scapularis, as a vector.
16 For scrub typhus, co-infection with
Leptospira species is likely common because outdoor activity is a shared risk factor for acquisition of these diseases.
17 To the best our knowledge, there have been no previous reports of co-infection of HGA and scrub typhus or HFRS. Scrub typhus is the most common rickettsial disease in Korea,
9 and HFRS is also a common endemic zoonosis.
14 Although the major vectors and route of transmission are different, there is possibility of co-infection with these diseases because of shared risk factor for exposure to these pathogens in similar environments. There are similar animal hosts between HGA and HFRS.
A. phagocytophilum have been detected in ticks including
H. longicornis,
Ixodes nipponensis, and
I. persulcatus and in wild animals such as striped field mice and the major host of
Hantan virus is wild rodents (
Apodemus species).
6
Compared to scrub typhus or HFRS, clinicians in Korea are largely unfamiliar with HGA. Therefore, many cases of HGA could have been missed, leading to underreporting.
The number of HGA cases in Korea has increased steadily since the first case was reported in 2013. Clinicians should consider the possibilities of HGA and co-infection in acute febrile patients after tick bites. It is likely that there will be more patients with HGA in the future, necessitating an active laboratory diagnosis and epidemiological investigation for this disease.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download