INTRODUCTION
PATHOGENESIS
1. Abnormal genetic mutation.
2. Distal aortic arch underdevelopment due to reduced anterograde intrauterine blood flow leading to underdevelopment of the fetal aortic arch.
3. Aberrant PDA tissue extrusion into the wall of the fetal thoracic aorta.
NATURAL HISTORY
PATHOPHYSIOLOGY
CLINICAL PRESENTATION
EVALUATION
IMAGING
Chest radiography
Echocardiography
 | Figure 3(A) A two-dimensional TTE from the suprasternal view demonstrating a discrete narrowing just distal to the take-off of the left subclavian artery (arrow) with continuous high velocity color Doppler signal across the coarctation. (B) Continuous wave Doppler TTE showing continuation of anterograde flow during diastole and peak pressure gradient of 60 mmHg across the coarctation.TTE = transthoracic echocardiography.
|
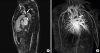
Cardiac magnetic resonance imaging
Computed tomography
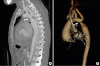 | Figure 5(A) Computed tomographic angiogram is revealing coarctation of the aorta just at the origin of the left subclavian artery (arrow). (B) 3D volume rendered reconstruction of the same patient revealing the coarctation (arrow), note the incidental finding of persistent left superior vena cava to left atrium. |
Catheter angiography
INDICATIONS FOR INTERVENTION
TREATMENT OPTIONS
Surgical management
1. Resection with end-to-end anastomosis. The first surgical therapy documented for CoA was performed through a lateral thoracotomy and involved resection of the coarcted segment followed by a direct suture anastomosis of the transected ends. High re-CoA rates complicated this approach due to the circumferential suture lines particularly when performed in neonates.
2. Patch aortoplasty. To minimize the concerns of re-CoA, patch augmentation, initially with Dacron, was used where the aorta was incised longitudinally through the coarcted segment on the lateral wall of the aorta and a prosthetic patch is sutured across the incision which enlarges the vessel diameter. This technique initially resulted in less frequent re-CoA compared to the end-to-end repair but it fell out of avour when aneurysms formed in 20–40% of cases on the opposite wall of the patch augmentation. Changing the patch material to polytetrafluoroethylene (PTFE) reduced the aneurysm formation to about 7% but still demonstrated a 25% risk of re-CoA. Patch aortoplasty is still being used but only in the context of complex arch reconstruction.18)
3. Subclavian flap aortoplasty. In this technique the subclavian artery (SCA) is ligated close to the origin of the left vertebral artery. The flap is generated by an incision of the SCA that is extended down onto the aortic isthmus and across the coarcted segment. The re-CoA rate of this technique seems to be relatively low when performed in older children up to 3%. However, when applied to neonates, re-CoA may occur in up to 23%.19) Although sacrificing the SCA does not result in left arm ischemia, it may cause claudication of the affected arm in the long term.
4. Extended end-to-end anastomosis. In contrast to a direct end-to-end anastomosis, the proximal clamp is placed across the aortic arch including the SCA or even the left carotid artery including the aortic arch. Distally, the aorta is clamped below the coarcted segment. After ligation and division of the ductus arteriosus, the coarcted segment is resected and the aortic arch is opened on its inferior aspect, followed by end-to-end anastomosis of the opened arch and the descending aorta. The procedure can be performed with low peri-operative mortality and reports show relatively low re-CoA rates of 4% to 13%.20)
5. Interposition graft. This technique has been reserved for patients in whom outgrowth of the graft is not a concern, or in patients with long-segment CoA. After the aorta is cross-clamped and the obstructive tissue resected, a tube graft of either aortic homograft or Dacron is sewn into the aorta, creating an unobstructed path for blood. The main disadvantage of this technique is that it requires a longer cross-clamp time for two surgical anastomoses to be sewn, and the tube graft will not grow with the patient. Yet, for adult-sized patients presenting with long-segment CoA, this technique may be preferable at many centers.
Catheter based repairs
Balloon angioplasty
Stenting
1) Stent types
Table 1
Characteristics of the different types of stents

2) Procedural steps
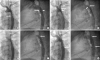 | Figure 6Angiogram in the transverse arch in left anterior oblique 30° (A) and straight lateral 90° (B) in a 28 years female patient with severe coarctation of the aorta, with a gradient of 30 mmHg. (A) The coarctation ridge (arrow). (B) Ductal diverticulum (long arrow) and the coarctation juxta ductal (shorter arrow). Note, there is a pacing catheter in the right ventricle. (C, D) Angiogram via side arm of delivery sheath (covered stent [Bentley 14 mm×39 mm] is uncovered half way). (C) Origin of the left subclavian artery (long arrow) and proximal tip of the stent (short arrow). (E, F) Angiogram via side arm of sheath after uncovering the stent completely. Note, origin of the left subclavian artery (arrow). (G, H) Angiogram via pigtail catheter in transverse arch after complete stent deployment showing good position (arrows) and remodelling of the area of coarctation. Residual gradient was 0 mmHg. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download