Abstract
Periprosthetic joint infections are a major cause of morbidity and mortality following total joint arthroplasty. Two-stage arthroplasty, with the use of an antibiotic cement spacer, is an effective means of managing periperiprosthetic joint infections. There is a lack of data relating to the management, prognosis, and clinical outcomes associated with multiple peri-prosthetic joint infections. Here, we present a case report of a patient successfully treated for three synchronic peri-prosthetic joint infections of both knees and a single hip.
Periprosthetic joint infection (PJI) is a rare but serious complication associated with both primary and revision total joint arthroplasty (TJA). The prevalence of PJI in primary hip and knee procedures is estimated to be between 0.5% and 3%1). While infection rates in primary arthroplasties remain low, PJI is the third most common reason for prosthesis failure and need for revision surgery2). As the number of primary hip and knee arthroplasties continues to rise, the number of periprosthetic infections can also be expected to increase3).
The current standard of care in the management of PJI is two-stage arthroplasty, which involves periprosthetic resection, introduction of an antibiotic cement spacer (ACS), followed by eventual re-implantation of a prosthesis145). Multiple studies have characterized the systemic complications of two-stage arthroplasty for primary PJIs (e.g., elevation in hepatic enzymes, allergic reactions, acute kidney injury [AKI]-which has been shown to occur in up to 17% of patients)5). In 1991, Murray et al.6) defined metachronic PJI as the spread of infection from one TJA to another separate arthroplasty and demonstrated that patients with multiple periprosthetic joints are at elevated risk of metachronic PJI after the development of one PJI. Nevertheless, there is an overall paucity of data available on the clinical outcomes of patients with synchronic PJI.
Here, we present a case of a patient with three synchronic PJIs who was treated with ACS; the patient experienced eventual eradication of infections and successful re-implantations. The patient did develop a post-operative AKI, which resolved by final follow up. To our knowledge, this is the first case report describing the presentation, post-operative course, and relevant literature for a patient treated with ACS for three synchronic hardware infections.
A 71-year-old male with a history of hyperlipidemia, sick sinus syndrome status post pacemaker placement, recent history of bacteremia, and remote history bilateral TKAs and right hip arthroplasty presented to the Emergency Department (ED) with left knee pain and inability to ambulate for two days. AP and lateral radiographs of the left knee at initial presentation are shown in Fig. 1A, B. Approximately 6 weeks prior to presentation, the patient was admitted for methicillin-resistant Staphylococcus aureus (MRSA) endocarditis and underwent removal of his pacemaker. The patient was placed on a 6-week course of ceftaroline and discharged to an acute rehab facility.
On presentation to the ED, his exam revealed an erythematous, swollen left knee with pain elicited on passive range of motion. Inflammatory markers were significant with a white blood cell count of 7.0×103/µL (normal, 5×103/µL to 10×103/µL) and an erythrocyte sedimentation rate of 77 mm/hr (normal, 0–20 mm/hr). A C-reactive protein level was 24.58 mg/L (normal, 0.00–7.48 mg/L). Arthrocentesis of the left knee revealed gram positive cocci and a synovial white cell count of 83,000 cells/mL. Preliminary cultures of the synovial fluid grew S. aureus and blood cultures were ultimately positive for MRSA. The patient was diagnosed with PJI using the Musculoskeletal Infection Society (MSIS) criteria and was started on weight-adjusted intravenous (IV) vancomycin as well as oral rifampin78). Vancomycin was dosed at 1 g every 12 hours.
On day two of admission, the patient developed pain in his contralateral knee and right hip. He was taken to the operating room for debridement and explanation of his left knee prosthesis and placement of a static antibiotic spacer using 120 g of bone cement (3 bags of DePuy MV bone cement; DePuy Synthes, Raynham, MA, USA) hand mixed with 9 g of vancomycin and 10.8 g of tobramycin. The decision was made to perform intraoperative aspiration the contralateral knee and right hip to rule out additional septic joints. Serum creatinine (Cr) was 1.0 mg/dL and blood urea nitrogen (BUN) was 19 mg/dL on the day of surgery. Intraoperative aspirations of the right knee and hip were positive for MRSA. The patient was subsequently returned to the operating room for explanation and placement of an antibiotic spacer in his right knee on post-operative day (POD) 2 and his right hip on POD 6. Identical formulations were used for each ACS preparation. Post-operative radiographs are provided in Fig. 2.
The patient was made non-weight bearing to his bilateral lower extremities post-operatively. Range of motion of the right hip under strict posterior hip precautions was allowed on the right side; no knee range of motion was allowed bilaterally. Throughout the postoperative course, the patient remained afebrile with a normal white blood cell. The post-operative course was complicated by elevated Cr of 1.46 mg/dL and BUN of 24 mg/dL on POD 8. Vancomycin trough level at that time was found to be elevated (29.4 µg/mL; normal, 10–20 µg/mL) and the next dose was held. Repeat vancomycin trough approximately 24 hours later was 22.9 µg/mL leading to discontinuation of vancomycin and initiated on IV daptomycin.
Despite discontinuation of vancomycin and adequate fluid resuscitation, Cr levels continued to rise. Cr levels peaked on POD 13 at 3.69 mg/dL. On POD 9, a random tobramycin level was drawn to determine if antibiotic elution from the cement spacers was occurring and found to be 10.4 µg/mL (normal reference, 0.57–1.30 µg/mL). Hemodialysis (HD) was started on POD 14. The patient received HD for a total of 13 days, after which he was transitioned to peritoneal dialysis for a total of 64 days with complete recovery of renal function and normalization of serum Cr levels.
Three months after resolution of the patient's AKI and infection, the patient returned for a right hip replantation without complication. Pre-operative labs demonstrated a Cr of 1.05 mg/dL and a BUN of 19 mg/dL. Bilateral knee replantation arthroplasties were performed 6 weeks later. Post operatively, the patient was made weight bearing as tolerated to the bilateral lower extremities and was advanced with physical therapy.
At one-year follow-up, the patient's bilateral TKA's and right THA were well positioned and revealed no evidence of infection. The patient was continued on suppressive antibiotics with minocycline 100 mg twice daily. Of note, long-term antibiotic suppression therapy was instituted due to concerns for recurrence of infection in a medically compromised patient. On physical examination, the patient was noted to have approximately 20° flexion contractures of his bilateral knees and continued to attend regular physical therapy. He was ambulating with assistance. Renal function was normal.
PJI, as defined by MSIS criteria in this report, are a potentially devastating complication of TJA78). Although there is abundant literature available on primary PJI, the risk factors and clinical outcomes for multiple PJI are less well understood91011). A limited set of case series have estimated the risk of multiple PJI to be between 6.3% to 20% after the development of an index infection in patients with multiple TJA611121314). Acute hematogenous spread has been described as the most likely mode of secondary infection in patients with metachronic or synchronic PJI1415). Haverstock et al.14) showed that acute hematogenous spread was implicated in approximately 50% (6/13) of patients with multiple PJI in their study population. More recently, Abblitt et al.15) revealed that bacteremia specifically is a significant risk factor for multiple PJI. A separate case series by Murray et al.6) and Luessenhop et al.12) demonstrated conflicting evidence on the association of rheumatoid arthritis and corticosteroid use with multiple PJI.
In patients with multiple PJI, the large majority of observed infections are likely to be metachronic rather than synchronic1112131415). For example, Jafari et al.13) retrospectively observed a population with multiple PJI and found a mean time to second infection of 2.0 years (range, 0–6.88 years) with only 3% of patients in the study suffering from synchronic PJI. Given the comparatively higher prevalence of metachronic versus synchronic PJI, most of our understanding of synchronic infections comes from literature that combines metachronic and synchronic infections. As such, although both presentations are hypothesized to have a similar pathophysiology, it is difficult to draw definitive conclusions about synchronic infections from the current body of literature.
Two-stage arthroplasty is the current standard of care in the treatment of either single or multiple joint PJI as it allows for the delivery of a high local concentration of antibiotics145). In 2004, Springer et al.16) showed that high-dose antibiotic cement was clinically safe and the use of the two-stage arthroplasty technique has been shown to result in both improvement of functional outcomes and successful eradication of infection in more than 90% of cases51718).
Re-infections following two-stage arthroplasty have been estimated to be as high as 37%; however, a more recent systematic review performed by Kunutsor et al.19) reported lower re-infection rates (between 7.2% and 10.6%)5). To our knowledge, there is no available data on clinical outcomes, including re-infection rates, associated with two-stage arthroplasty for the treatment of metachronic or synchronic PJI.
The reported mortality rate from PJI ranges from 2.7% to 18%920). A five-fold increase in mortality rate at one-year post-operatively has been reported in cases of revision TJA secondary to PJI as compared with revision due to aseptic failure21). Sepsis is a major contributor to mortality in patients with PJI, however there also appears to be a trend toward increased mortality in patients with other medical comorbidities, particularly cardiac disease, AKI, and MRSA infections2122). Additionally, periprosthetic joints infected with MRSA, as in our current case, are associated with increased rates of subsequent PJI in the same joint or a second joint as well as higher mortality, poor outcomes, and inflated health care costs22).
Despite multiple significant medical comorbidities and three synchronic PJIs with MRSA, the patient's bilateral TKAs and right THA showed no evidence of infection and kidney function remained normal at final follow up. Nevertheless, AKI represents an important complication of two-stage arthroplasty. A retrospective study done by Menge et al.23) revealed that a higher dose of antibiotics in an ACS is positively associated with an increased risk of AKI. It been also been shown that AKI is independently associated with increased mortality, length of stay, and increased cost in hospitalized patients24). We theorize that the observed AKI was multifactorial secondary to IV vancomycin, age-related decline in baseline renal function, and the observed antibiotic elution from the implanted spacers.
While the risk of high-dose antibiotics must ultimately be weighed against risks of inadequate treatment of an ongoing infection, as this case suggests, two-stage arthroplasty using high doses of antibiotics is likely to be necessary to adequately treat PJIs given the significant morbidity and mortality associated with an infected prosthesis. Although the patient was unable to regain pre-operative levels of function, the patient was successfully reimplanted and was ambulatory and progressing with physical therapy at most recent follow up (Fig. 1, 2, 3). As cases of multiple PJI increase, further research is necessary to risk stratify patients and understand the complications of both metachronic and synchronic PJI in order to ensure satisfactory clinical outcomes.
Figures and Tables
Fig. 1
(A) Anteroposterior (AP) and lateral views of right total knee arthroplasty at initial presentation. (B) AP and lateral views of the left total knee arthroplasty at onset of symptoms. (C) AP view of the right total hip arthroplasty at the onset of symptoms.
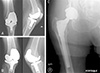
Fig. 2
Anteroposterior (AP) radiograph of the bilateral knee and AP radiograph of the right hip after component explanation with antibiotic cement spacer placement.
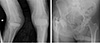
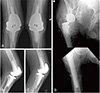
Fig. 3
One-year post-operative radiographs. (A) Anteroposterior (AP) view of bilateral revision total knee arthroplasty. (B) Lateral view of left revision total knee arthroplasty. (C) Lateral view of right revision total knee arthroplasty. (D) AP views of the right hip and right femur revealing complete revision right total hip arthroplasty stem.
References
1. Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007; 89:871–882.


2. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010; 468:45–51.


3. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005; 87:1487–1497.

4. Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983; 65:1087–1098.


5. Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty. 2013; 28:1490–1498.e1.

6. Murray RP, Bourne MH, Fitzgerald RH Jr. Metachronous infections in patients who have had more than one total joint arthroplasty. J Bone Joint Surg Am. 1991; 73:1469–1474.


7. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011; 469:2992–2994.

8. Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013; 56:1–10.

9. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998; 27:1247–1254.


10. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008; 466:1710–1715.



11. Clesham K, Hughes AJ, O'hEireamhoin S, Fleming C, Murphy CG. Second-site prosthetic joint infection in patients with multiple prosthetic joints. Eur J Orthop Surg Traumatol. 2018; 28:1369–1374.


12. Luessenhop CP, Higgins LD, Brause BD, Ranawat CS. Multiple prosthetic infections after total joint arthroplasty. Risk factor analysis. J Arthroplasty. 1996; 11:862–868.

13. Jafari SM, Casper DS, Restrepo C, Zmistowski B, Parvizi J, Sharkey PF. Periprosthetic joint infection: are patients with multiple prosthetic joints at risk? J Arthroplasty. 2012; 27:877–880.


14. Haverstock JP, Somerville LE, Naudie DD, Howard JL. Multiple periprosthetic joint infections: evidence for decreasing prevalence. J Arthroplasty. 2016; 31:2862–2866.


15. Abblitt WP, Chan EW, Shinar AA. Risk of periprosthetic joint infection in patients with multiple arthroplasties. J Arthroplasty. 2018; 33:840–843.


16. Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004; (427):47–51.

17. Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma. 2004; 56:1247–1252.


18. Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995; 77:1576–1588.


19. Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD. INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One. 2016; 11:e0151537.

20. Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003; 36:1157–1161.


21. Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013; 95:2177–2184.


22. Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty. 2010; 25:6 Suppl. 103–107.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download