This article has been
cited by other articles in ScienceCentral.
Dear Editor:
The dermal papilla (DP) of hair follicle is essential for hair morphogenesis, growth, and regeneration. Therefore, DP cells are considered to be an optimal cell source for genesis of new hair follicles
12. However, there remains an experimental challenge resulting from DP cells gradually losing their hair-inductive capacity when cultured two-dimensionally (2D)
3. Three-dimensional (3D) spheroid culturing was successfully employed to overcome the loss of hair inductivity of 2D-cultured human DP cells
456.
STAT5 is a signal transducer and activator of transcription (STAT). Recently, Legrand et al.
7 reported that the actived form of STAT5 (phospho-STAT5; P-STAT5) is restricted to the DP cells and activation of STAT5 in the DP plays an important role in hair growth phase induction. They showed that hair-inductive capacity of mouse DP-derived multipotent stem cells, skin-derived precursors, is significantly enhanced by adenoviral overexpression of STAT5A or STAT5B
7. In line with this, STAT5 deletion impaired formation of
de novo hair follicles in skin-derived precursors
7. In contrast, Harel et al.
8 reported that the hair inductivity of human DP spheres is enhanced by the treatment with tofacitinib, a STAT signaling inhibitor. These controversial reports prompted us to investigate role of STAT5 in the hair inductivity of human DP cells. We performed STAT5 knock-down in human DP spheres and the spheres were implanted into the back of the nude mice together with mouse epidermal cells.
The Medical Ethical Committee of the Kyungpook National University Hospital (Daegu, Korea) approved all of the described studies (IRB no. KNUH 2013-02-001-007). Student's t-test was used to analyze differences between groups using Microsoft Excel. We considered
p-values <0.05 to be statistically significant. We first verified whether STAT5 expression and that of its known downstream transcriptional targets, SOCS2 and SOCS3, is higher in the 3D human DP spheres, compared with the expression of these transcripts in the 2D-cultured human DP cells. Expression of STAT5A, STAT5B, SOCS2, and SOCS3 was significantly upregulated in the DP spheres as examined by qPCR analysis (
Fig. 1A). Next, to address the functional role of STAT5 in the hair inductivity of DP spheres, 2D-cultured DP cells were transfected with STAT5A siRNA, or STAT5B siRNA for 48 hours, before being harvested and reseeded to form STAT5 knock-down spheres. The spheroid formation was induced for 48 hours in each well of 96-well HydroCell plates (10
4 cells per well) before implantation. The sphere blocks were cut to 7-µm thick sections on glass slides. The slides were incubated with a rabbit monoclonal antibody against phosphorylated STAT5A/B (1:100 dilution; Santa Cruz, CA, USA), washed with phosphate-buffered saline, and incubated with Alexa Fluor 488-labeled donkey anti-rabbit secondary antibody (1:100 dilution; Molecular Probes, Eugene, OR, USA). The slides were then incubated with 4′,6-diamidino-2-phenylindole for nuclear staining. The P-STAT5 expression was markedly reduced in STAT5 (A or B) knock-down DP spheres, when compared to that in control DP spheres as confirmed by immunochemical analysis (
Fig. 1B).
An established patch hair reconstitution assay was employed to assess the hair-inductive capacity of three kinds of DP spheres
9. Implantation was performed as described previously
46. Briefly, freshly isolated neonatal mouse epidermal cells were combined with control or STAT5A, or STAT5B siRNA-transfected DP spheres (100 spheres) and co-transplanted subcutaneously into the skin on the back of the nude mice. To verify newly induced hair formation, skin samples were excised from the mice and examined after three weeks. Knock-down of STAT5 significantly decreased the hair inductivity of DP spheres (
Fig. 1C). Number of induced hair follicles was significantly reduced in STAT5A siRNA-transfected or STAT5B siRNA-transfected DP spheres compared to control siRNA-transfected DP spheres (
p<0.05). Control siRNA-transfected DP spheres induced 79±14 hair follicles, while STAT5A and STAT5B knock-down DP spheres induced 38±16 and 26±21 hair follicles, respectively. The results of patch assays are summarized in
Table 1. To confirm that the DP cells of the newly induced hair follicles were originated from human, cells were labelled with the fluorescent dye. As shown in
Fig. 1D, DP in the induced follicles contained dye-labelled human cells.
We further examined whether knock-down of STAT5 regulates the expression of DP signature genes, such as ALP and Wnt10b, using qPCR analysis. We found that mRNA levels of ALP and Wnt10b are significantly decreased in STAT5 siRNA-transfected DP spheres, compared to those in control DP spheres (
Fig. 1E). Considering that the hairinductive capacity of dermal mesenchymal components is closely correlated with the expression of ALP
10, significantly reduced hair inductivity in STAT5 knock-down DP spheres is validated by these observations. How STAT5 regulates mRNA levels of ALP and Wnt10b remains to further investigated.
In summary, our study demonstrates that expression of STAT5 and its target genes is elevated when 2D-cultured human DP cells are induced to form
in vivo-like DP spheroids. In addition, our hair reconstitution data demonstrate that enhanced hair-inductive capacity in DP spheres is at least partly due to enhanced STAT5 levels. Our data is in line with the findings of Legrand et al.
7 who reported impaired hair inductivity in STAT5-deleted skin-derived precursors.
Figures and Tables
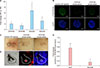 | Fig. 1Hair-inducing capacity of three-dimensionally (3D) cultured human dermal papilla cells is impaired by the knock-down of STAT5. (A) The expression of STAT5A, STAT5B, SOCS2, and SOCS3 in 3D dermal papilla (DP) spheres was quantitatively compared with that in two-dimensionally (2D) cultured DP cells by real-time polymerase chain reaction (PCR). Data are means±standard deviation (SD) of three independent experiments using DP cells of three different patients (*p<0.01). (B) Immunofluorescence staining of phospho-STAT5 (P-STAT5) (green; upper panels) and the corresponding 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue; bottom panels) in DP spheres are shown. Bar=0.1 mm. (C) Hair induction assay with three kinds of DP spheres (106 cells) in combination with freshly isolated mouse epidermal cells (106 cells). Bar=1 mm. (D) Phase-contrast and fluorescence imaging of DiI and DAPI in hair follicles induced from the DP spheres. Bar=0.1 mm. (E) The expression of ALP and Wnt10b in STAT5B knock-down DP spheres was quantitatively compared with that in control DP spheres using real-time PCR. Data are means±SD of three independent experiments using DP cells of three different patients (*p<0.01, **p<0.05). The sequences of primers used are as follows: STAT5A, 5′-GTAAGGCTGTGTACACTGACAC-3′ and 5′-CATAGGGTTCACAGAGAGTCTG-3′; STAT5B, 5′-GACTCAGAAATTGGCGGCATC-3′ and 5′-CGGTGTCAAGGACTGAGTCAG-3′; SOCS2, 5′-CCCGCAGGGACTCGTTTT-3′ and 5′-TTCGCGTCCTTCCTTGAAGT-3′; SOCS3, 5′-CAGCTCCAAGAGCGAGTACCA-3′ and 5′-AGAAGCCGCTCTCCTGCAG-3′; ALP, 5′-CTGGAACCGCACGGAACT-3′ and 5′-CTGCTTGGCTTTTCCTTCATG-3′; Wnt10b, 5′-CTCTGGGATGTGTAGCCTTC-3′ and 5′-GGCTCTGGAGTTGAGAAGTG-3′; and GAPDH, 5′-TGGAAATCCCATCACCATCTTC-3′ and 5′-CGCCCCACTTGATTTTGG-3′.
|
Table 1
Summary of patch hair reconstitution assay results


ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) 2016R1A2B4014786. This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2018R1A2A2A05018574).
References
1. Ohyama M, Zheng Y, Paus R, Stenn KS. The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol. 2010; 19:89–99.


3. Ohyama M, Veraitch O. Strategies to enhance epithelialmesenchymal interactions for human hair follicle bioengineering. J Dermatol Sci. 2013; 70:78–87.


4. Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012; 132:237–239.


7. Legrand JMD, Roy E, Ellis JJ, Francois M, Brooks AJ, Khosrotehrani K. STAT5 activation in the dermal papilla is important for hair follicle growth phase induction. J Invest Dermatol. 2016; 136:1781–1791.


8. Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015; 1:e1500973.

9. Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005; 124:867–876.


10. McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003; 121:1267–1275.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download