CASE REPORT
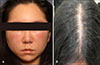
A 26-year-old woman with a history of female pattern alopecia presented to the dermatology clinic with edema, erythema, and numbness on her entire face after her first intralesional injection on the scalp of a mesotherapy mixture at a private clinic (
Fig. 1). She was healthy with no past medical history or atopic disease. According to her medical records from the clinic, a painful edema and redness on her forehead developed on the day after the injection and later extended downward to her entire face. Edema and pain persisted for more than a week, and finally atrophic scars with crusts were formed at each injection site (
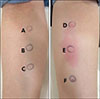
Fig. 2). An intradermal allergic skin test for possible culprit agents was performed, and a positive reaction to 0.25 ml triamcinolone acetonide (Dongkwang Pharm, Seoul, Korea) at the concentration of 10 mg/ml after 24 hours was identified (
Fig. 3). The patient was diagnosed with alopecia due to a delayed hypersensitivity reaction to intralesional triamcinolone acetonide injection. Our clinic prescribed treatment with 3% topical minoxidil and intermittent non-ablative fractional laser therapy for the regeneration of the scalp dermis, but no sufficient improvement was noticed. We received the patient's consent form about publishing all photographic materials.
DISCUSSION
In contrast to the extremely frequent application of corticosteroids in many dermatologic diseases, the reported incidence of allergic reactions to these compounds is relatively uncommon
1. Recently, however, the adverse events from the use of corticosteroids are increasingly recognized worldwide as a problem with considerable clinical importance. Both type I and type IV hypersensitivity reactions have been reported following exposure to corticosteroids
2. Especially, the allergic contact dermatitis after the topical application of corticosteroids is the most commonly observed hypersensitivity reaction, occurring in 4%~5% of the population
3.
Corticosteroids are also known to cause sensitization when used systemically, albeit this mode of sensitization is much more rare
4. The prevalence of IgE-mediated, type I hypersensitivity reactions to systemic corticosteroids is reported to be approximately 0.3%
5. One of the earliest reports of the immediate type I reaction to systemic corticosteroids described urticaria, angioedema, and bronchospasm after injections of corticosteroids in 20 of 2,256 patients
6.
A type IV hypersensitivity reaction to systemic and intralesional steroids presents typically as a generalized dermatitis or an exanthematous eruption, occasionally with blisters and purpuras
7. Bircher et al.
8 reported a delayed hypersensitivity to the oral administration of prednisone, inducing a generalized exanthem in the patient. Räsänen and Hasan
3 also documented delayed hypersensitivity reactions to oral or intralesional corticosteroids in five patients, who developed diffuse erythema in a few to 24 hours after patch or intradermal skin tests.
There are 5 classes of structurally distinct corticosteroids, termed as class A, B, C, D1, and D2
910. These groups were defined according to substitutions on the corticosteroid structure and clinical characteristics. However, less is known about the classification and cross-reactivity pattern of the less common reactions following systemic administration of corticosteroids
11. Our patient developed marked edema and erythema a few hours after the intralesional injection of triamcinolone acetonide, which belongs to the class B corticosteroids. We performed an allergic intradermal skin test for suspected drugs, including triamcinolone, the excipients (100,000:1 mixture of 2% lidocaine/epinephrine, sodium bicarbonate, and 0.9% sodium chloride), antibiotics (lincomycin), and dexamethasone. After 24 hours, the patient presented with a positive intradermal skin test result solely at the triamcinolone site. Although a strong positive reaction to triamcinolone was noted, the intradermal skin test for dexamethasone, which belongs to the class C corticosteroids, was negative. This result supports the recent study on reaction patterns to corticosteroids, which indicated that reactivity to only one type of corticosteroid is much more common than reactivity to multiple types
12. Therefore, even though a patient reports no adverse events with previous steroid use, a dermatologist should still consider the possibility that the patient may undergo a hypersensitivity reaction to other types of steroids.
Several previous reports on triamcinolone acetonide hypersensitivity have reported that the culprit agent could be not only the corticosteroid itself but also its excipients, such as carboxymethylcellulose, benzyl alcohol, and polysorbate 80
131415. As the limitation to our study, we did not extensively analyze the excipients added to the triamcinolone mesotherapy mixture. It is crucial to perform allergologic testing of individual pharmaceutical excipients as well, since allergic reactions are not purely caused by the active pharmaceutical constituents.
In conclusion, our report presents a rare case of permanent hair loss following a severe type IV delayed hypersensitivity reaction to intralesional triamcinolone acetonide as part of a mesotherapy mixture. Although multiple side effects have been reported, mesotherapy with a diverse mixture of unapproved products has gained popularity for the treatment of alopecia
16. To prevent irreversible side effects, physicians should always be fully aware that both IgE-mediated and non-IgE-mediated hypersensitivity reactions from the corticosteroids or their mesotherapy mixtures are possible. Especially, patients with atopic disease or previous history of drug allergy should be considered for testing to the corticosteroid as well as the excipients, because patterns of hypersensitivity reactions to mesotherapy are not fully uncovered at this point.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download