Abstract
Figures and Tables
Fig. 1
Rapidly growing crateriform tumors on the pretibial area (A), presternal (B) and parietal (C).

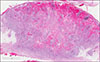
Fig. 2
Proliferating keratin-filled invagination of the epidermis with areas of deep penetrating strands (H&E, ×10).
Journal List > Ann Dermatol > v.31(2) > 1117348


Álvaro March-Rodriguez 
https://orcid.org/0000-0002-4637-7079
Beatriz Bellosillo 
https://orcid.org/0000-0002-5335-2726
Alberto Álvarez-Larrán 
https://orcid.org/0000-0001-6387-4619
Carles Besses 
https://orcid.org/0000-0003-1806-3440
Ramon M Pujol 
https://orcid.org/0000-0002-5622-6055
Agustí Toll 
https://orcid.org/0000-0003-2656-0076