Abstract
Purpose
We sought to determine the factors associated with the severity of aggravated hand lesion in patients with diabetes mellitus (DM).
Methods
DM patients with hand lesions ranging from non-suppurative/suppurative to gangrenous (which require surgical treatment) were selected for analysis. Between January 2008 and June 2016, 216 patients with signs of of redness, swelling, and pain with lesions between the fingertip and wrist were analyzed retrospectively. Patients were grouped according to whether they received conservative treatment (group 1) or operative treatment (group 2), and univariate and multivariate analyses were performed according to demographic, laboratory data, co-morbidities, and method of diabetic treatment in both groups.
Results
Age, duration of the morbidity, gender, smoking, co-morbidities, body mass index, other laboratory findings, onset time before treatment, and the presence/classification of trauma history, were all not significant. However, Hb1Ac was found to be 5.96%±0.80% and 8.01%±0.82% in group 1 and 2 respectively, which differed significantly (OR=58.5, p<0.001).
Conclusion
It is possible to manage hand lesions in DM patients with a variety of methods, ranging from conservative to surgical treatment. HbA1c level was determined to be the most important contributing factor in selection of the more rigorous surgical treatment. Moreover, it was determined that even subtle lesions should not be neglected in DM patients as they are susceptible to rapid progression if left untreated.
Figures and Tables
 | Fig. 1(A) A 56-year-old woman (group 2) presented with spontaneous discharge on the palm of the left hand without any definitive injury or observed trauma. She was diagnosed with type II diabetes mellitus 4 years ago, and the HbA1C was 9.2% at presentation. (B) Incision and drainage (I and D) under the brachial plexus block in a gangrenous hand was performed and the wound was left open. Two days after or mean±standard deviation., the tissue defect became well demarcated. (C) During hospitalization, I and D was carried out twice a day and intravenous antibiotic were administered. (D, E) After 23 days of wound management, the amount of discharge decreased significantly. (F) Patient follow-up two years after I and D showed that the hand was free from any infectious symptoms and signs. Scarring was visible on the palms and the dorsum. At this point in time, HbA1c levels had returned to within normal ranges. |
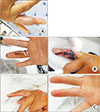 | Fig. 2(A) A 32-year-old male (group 2) complained of painful swelling on the right 4th finger. He was diagnosed as type I diabetes mellitus (DM) 17 years ago. However, the condition was not managed properly. At presentation, HbA1c was 8.6%. (B) The wound was opened one day after Incision and drainage. (C) After daily irrigation with intravenous antibiotics during a 16-day period of hospitalization, the condition of the finger was determined to be satisfactory and the symptoms of DM were found to be under control. |
 | Fig. 3A 66-year-old male (group 2) complained of painful swelling on the dorsum of the right hand. He was diagnosed as type II diabetes mellitus 12 years ago. The condition was not managed properly. HbA1c was 7.7% at time of presentation. Skin from the antecubital region was obtained for a skin graft procedure. |
ACKNOWLEDGEMENTS
This research was supported by Chungnam National University Hospital Research Fund, 2017.
References
2. Hinchcliffe RJ, Andros G, Apelqvist J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab Res Rev. 2012; 28:Suppl 1. 179–217.


3. Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007; 4:286–287.


4. Armstrong DW, Tobin C, Matangi MF. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Can J Cardiol. 2010; 26:e346–e350.

5. Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Technol. 2011; 5:1591–1595.



6. American Diabetes Association. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. 1995; 18:1415–1427.

7. Boreland L, Scott-Hudson M, Hetherington K, Frussinetty A, Slyer JT. The effectiveness of tight glycemic control on decreasing surgical site infections and readmission rates in adult patients with diabetes undergoing cardiac surgery: a systematic review. Heart Lung. 2015; 44:430–440.


8. Bartelink ML, Hoek L, Freriks JP, Rutten GE. Infections in patients with type 2 diabetes in general practice. Diabetes Res Clin Pract. 1998; 40:15–19.


9. Truntzer J, Vopat B, Feldstein M, Matityahu A. Smoking cessation and bone healing: optimal cessation timing. Eur J Orthop Surg Traumatol. 2015; 25:211–215.


10. Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fc-gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS One. 2014; 9:e92977.

11. Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006; 55:487–495.


12. Price CL, Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2010; 14:1806–1815.


13. Ilyas R, Wallis R, Soilleux EJ, et al. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011; 216:126–131.


14. Stegenga ME, van der Crabben SN, Blümer RM, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008; 112:82–89.



15. Richmond NA, Maderal AD, Vivas AC. Evidence-based management of common chronic lower extremity ulcers. Dermatol Ther. 2013; 26:187–196.


16. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002; 287:2570–2581.

17. Wagner FW. A classification and treatment program for diabetic, neuropathic, and dysvascular foot problems. Instr Course Lect. 1979; 28:143–165.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download