Abstract
Background
Postoperative nausea and vomiting (PONV) frequently occurs following bimaxillary orthognathic surgeries. Compared to opioids, Nefopam is associated with lower incidences of PONV, and does not induce gastrointestinal tract injury, coagulopathy, nephrotoxicity, or fracture healing dysfunction, which are common side effects of Nonsteroidal anti-inflammatory drugs. We compared nefopam- and fentanyl-induced incidence of PONV in patients with access to patient-controlled analgesia (PCA) following bimaxillary orthognathic surgeries.
Methods
Patients undergoing bimaxillary orthognathic surgeries were randomly divided into nefopam and fentanyl groups. Nefopam 120 mg or fentanyl 700 µg was mixed with normal saline to a final volume of 120 mL. Patients were given access to nefopam or fentanyl via PCA. Postoperative pain intensity and PONV were measured at 30 minutes and 1 hour after surgery in the recovery room and at 8, 24, 48, and 72 hours after surgery in the ward. The frequency of bolus delivery was compared at each time point.
Results
Eighty-nine patients were enrolled in this study, with 48 in the nefopam (N) group and 41 in the fentanyl (F) group. PONV occurred in 13 patients (27.7%) in the N group and 7 patients (17.1%) in the F group at 8 hours post-surgery (P = 0.568), and there were no significant differences between the two groups at any of the time points. VAS scores were 4.4 ± 2.0 and 3.7 ± 1.9 in the N and F groups, respectively, at 8 hours after surgery (P = 0.122), and cumulative bolus delivery was 10.7 ± 13.7 and 8.6 ± 8.5, respectively (P = 0.408). There were no significant differences in pain or bolus delivery at any of the remaining time points.
Postoperative nausea and vomiting (PONV) is the most common postoperative complication after orthognathic surgery. Forty percent of patients experience PONV within 24 hours after surgery [1]. To maintain the postoperative occlusion, intermaxillary fixation is performed using an elastic band or wire. In this condition, the risk of airway obstruction is increased because it is difficult to effectively remove intra-oral secretions and bleeding [2]. Therefore, control of PONV is essential.
Bimaxillary orthognathic surgeries are associated with a higher level of pain compared to other oral and maxillofacial procedures [3]. Patient-controlled analgesia (PCA) is widely used for pain control after orthognathic surgeries [45]. PCA using a microprocessor-controlled infusion pump is highly effective in lowering patient anxiety caused by the gap between the patient's pain recognition and time of analgesic administration [6]. Intravenous PCA is used with several types of narcotic analgesics such as morphine, fentanyl, pethidine, piritramide, nalbuphine, and tramadol [7]. These opioids increased the risk of nausea, vomiting, and respiratory depression in some studies [8].
Nefopam is associated with lower incidences of gastrointestinal tract injury, coagulopathy, antipyretic effect, and nephrotoxicity, which are known side effects of Nonsteroidal anti-inflammatory drugs (NSAID) [91011121314]. Recently, clinical use of the nonopioid agent nefopam has increased due to relatively higher safety on hemorrhage, infection, and nephrotoxic patients. Nefopam has also been reported to decrease respiratory depression and PONV, which are adverse effects of opioids [151617]. Furthermore, recent reports suggested that NSAIDs may impede bone healing after orthopedic surgeries [1819], and nefopam does not induce this side effect [20].
This study aimed to investigate whether using nefopam to control postoperative pain following orthognathic surgeries lowered the incidence of PONV with similar pain control effects as fentanyl.
We conducted a single-center, prospective, randomized, double-blind study from August 2015 to December 2017 at Seoul National University Dental Hospital, Republic of Korea. Permission to conduct this study was granted by the institutional review board of Seoul National University Dental Hospital (approval number, CME15001). Written informed consent was obtained from all participants. All aspects of participant privacy and confidentiality were preserved. The investigation was registered with the Clinical Research Information Service (https://cris.nih.go.kr/KCT0001592) and performed according to the guidelines for the proper conduct of medical research on human participants.
Patients undergoing bimaxillary orthognathic surgery with intravenous PCA for postoperative pain control were enrolled. All patients had an American Society of Anesthesiologists (ASA) patient status of I or II and were between 20 and 40 years of age. Exclusion criteria were as follows: (1) urgent or emergent case, (2) re-do case, (3) ASA status of at least III, (4) allergic to fentanyl or nefopam, (5) history of drug abuse, (6) chronic pain (≥ 3 months), (7) use of analgesic or hypnotic medication within 2 weeks, (8) hepatic, renal, or cardiac insufficiency, (9) pulmonary diseases such as chronic obstructive pulmonary disease, asthma, and upper respiratory infection within 2 weeks, (10) smokers, (11) pregnant or breastfeeding, (12) refused to participate, (13) diabetes or neuropathic diseases, (14) unable to use the PCA device, (15) others who the investigator judged to be inappropriate candidates for participation in the clinical study. All patients received a general explanation of the study process, including instruction in the use of the visual analogue scale (VAS) ranging from 0 (no pain) to 10 (worst pain imaginable). The patients were also carefully instructed in use of the Accumate® 1100 PCA device (Wooyoung Medical Co. Ltd., Jincheon, Korea) that was used in the study.
The patients were randomly assigned to one of two groups using a computer-generated random number table (available at http://www.randomization.com/). The patients were allocated to receive either nefopam-based (nonopioid; N group) or fentanyl-based (F group) PCA for postoperative pain control using sealed envelopes containing the treatment options (N and F) before induction of anesthesia. All anesthesiologists, surgeons, nurses, and patients were blinded to the study. All data were collected by trained observers who also were blinded and did not participate in patient care.
All patients arrived at the operation room without premedication and were not given any preanesthetic medications. Anesthesia was induced after establishing routine patient monitoring (pulse oximetry, electrocardiography, and noninvasive blood pressure monitoring) and bispectral index monitoring. Following preoxygenation, anesthesia was induced with a 5 µg/mL effect site concentration of propofol and 5 ng/mL of remifentanil using an Orchestra Base Primea target-controlled infusion system (Fresinius Kabi, Bad Homburg, Germany). Anesthesia was maintained with propofol (2–4 µg/mL) and remifentanil (3–10 ng/mL) depending on bispectral index level (bispectral index was maintained between 40–60) and vital signs. Rocuronium (0.6 mg/kg) was administered for muscle relaxation following anesthesia induction and additional doses were administered as necessary (self-respiration is restored or the operator wished). After nasotracheal intubation, patients were ventilated with 50% oxygen in air. The tidal volume was 6–8 mL/kg (lean body mass), and positive end expiratory pressure was utilized as necessary. Respiratory rate was adjusted to maintain end-tidal carbon dioxide partial pressure from 30 to 35 mmHg. Invasive blood pressure monitoring and arterial blood gas analysis were performed by placing the catheter on the dorsalis pedis artery. Upon completion of the operation, residual neuromuscular paralysis was reversed using sugammadex (200 mg) and the PCA (Group N, 120 mg nefopam; Group F, 700 mcg fentanyl in total normal saline 120 mL) machine was connected. After recovery of self-respiration, patients were transferred to a postanesthetic care unit. After confirmation of complete recovery of consciousness, self-respiration (with sustained spontaneous respiration rate > 12/min), and an open airway, the tracheal tube was removed.
Twenty milligrams of nefopam provides an analgesic effect equivalent to 6–12 mg morphine, and 10 mg of morphine provides equivalent analgesia to 100 µg fentanyl [21]. Therefore, PCA was set as follows: 120 mg of nefopam for the N group and 700 µg of fentanyl for the F group in total volumes of 120 mL with normal saline. Continuous basal infusion of nefopam or fentanyl was provided (1 mL/hour). The PCA machine allowed for a 1 mL bolus, with a 15 minutes lockout time, and a 5 mL maximum per hour. Drugs were infused using an Accumate 1100®. The PCA device time was synchronized with Korea Standard Time at the national metrology institute. All labels on the PCA devices were hidden so that patients and medical staff who had direct contact with the patients did not know which drug was being administered. If postoperative pain was not controlled by continuous PCA infusion, ketorolac was administered intravenously as a rescue analgesic. If PONV occurred, antiemetics were administered.
The primary outcome was the incidence and number of PONV events after surgery. PONV was assessed using a 4-point ordinal scale (0 = none, 1 = nausea, 2 = retching, 3 = vomiting) upon arrival at the Post-anesthesia care unit 0.5, 1, 8, 24, 48, and 72 hours after surgery. Nausea was defined as a subjectively unpleasant sensation associated with awareness of the urge to vomit. Retching was defined as labored, spasmodic, rhythmic contraction of the respiratory muscles without expulsion of gastric contents. Vomiting was defined as the forceful expulsion of gastric contents from the mouth.
The secondary outcomes were pain score, sedation, shivering, difficulty sleeping, and other unwanted symptoms. Pain was assessed using the visual analogue scale (VAS) ranging from 0 (no pain) to 10 (worst pain imaginable) at 0.5, 1, 8, 24, 48, and 72 hours after surgery, both at rest (VASr) and during movement (VASm). Total PCA volume, need for rescue analgesics, and dose of rescue analgesics and antiemetic injections were recorded at 0.5, 1, 8, 24, 48, and 72 hours after surgery. The PCA discontinued if the respiratory rate was < 12/min, oxygen saturation was < 95%, sedation score was ≥ 4, or when other side effects caused by the PCA were suspected. If use of the PCA was discontinued, the reason for discontinuation, and the time at which discontinuation occurred was noted by medical personnel who did not have information about this study. The occurrence of side effects was assessed by checking for sedation, shivering, difficulty in sleeping, constipation, hypotension, and other unwanted symptoms for 72 hours postoperatively. Degree of sedation was scored as follows using the Ramsay sedation assessment scale [22]: 1 = anxious, agitated, or both; 2 = cooperative, oriented, and tranquil; 3 = responds to commands only; 4 = a brisk response to a light glabellar tap; 5 = a sluggish response to a light glabellar tap; 6 = no response. Patient satisfaction scores were also evaluated using a 5-point Likert scale. The Likert scale was scored as follows: 1 = not at all satisfied; 2 = slightly satisfied; 3 = moderately satisfied; 4 = very satisfied; 5 = completely satisfied. All outcomes were collected and analyzed by a biostatistician who was not a participant in the study and only knew details of study design.
After the IV PCA device was collected, information regarding patient self-administration (the time of starting the device, the number of bolus attempts, the amount of bolus drug delivery, and the end of device usage) was downloaded to personal computers loaded with an IV PCA device-specific download program, Eventlog Viewer version 1.1 for Accumate1100 (Wooyoung medical, Inc., Seoul, Korea).
To determine appropriate sample sizes, we assumed that incidences of PONV in the F group and the N group of 25% and 10%, respectively, would be significant on the basis of previous publication [16]. Seventy-nine patients per group were required for a power of 80% with a type I error rate of 5%. Considering a drop-out rate of 10%, 174 patients were planned to be included. However, interim analysis after including 89 patients showed a high incidence of PONV in the N group, and the study was stopped.
Measured values were presented as mean ± SD or as a number (%). Intra-group distributions of variables were evaluated for normality using the Kolmogorov-Smirnov and histogram tests. Fisher's exact test or χ2 test was used to evaluate categorical variables and Student's t-test or Mann-Whitney U test were used to evaluate continuous variables. P < 0.05 was considered statistically significant. SPSS version 25.0 for Windows was used for statistical analyses.
Eighty-nine patients were examined from August 2015 to December 2017, with 48 in the nefopam (N) group and 41 in the fentanyl (F) group. During the 72-h period after surgery, 7 patients in the N group and 7 patients in the F group were discharged, and thus lost to follow-up (Fig. 1). Baseline demographic and perioperative characteristics are shown in Tables 1 and 2, respectively, and there were no significant differences between the two groups. Table 3 shows the incidence of PONV at each time point. In contrast to our hypothesis, in the interim survey of 89 participants, the incidence of PONV was higher in the N group (n = 13, 27.7%) than in the F group (n = 7, 17.1%; P = 0.568) at 8 hours after surgery, so we stopped the study.
Postoperative pain intensity was measured using VAS (Table 4), with no significant differences between the two groups. In addition, there were no significant differences in total bolus attempts, bolus delivery, and infused volume, all of which were collected from the PCA device at all time points (Table 5). The incidence of use postoperative rescue analgesics and antiemetics at each time point is shown in Table 6, and there were no significant differences between the two groups. The incidence of adverse events and patient satisfaction at different time points following bimaxillary orthognathic surgery in patients receiving PCA with nefopam or fentanyl is shown in Table 7. There were significant differences in patient satisfaction at 24 and 48 hours after surgery between the two groups, with no significant differences for other parameters.
Postoperative pain is the greatest fear of patients planning to undergo bimaxillary orthognathic surgery. In general, control of postoperative pain is accomplished by PCA, which can be controlled by patients with low side effects. Opioids are the most common drugs used for PCA [523], and opioids are either used alone or are mixed with NSAIDs [4]. With PCA, postoperative pain intensity is known to decrease to an average VAS score of 4 or below by 8 hours after surgery and to a VAS score of 3 or below by 24 hours after surgery [4]. The amount of analgesics required to control pain following bimaxillary orthognathic surgery has been shown to be less than the amount required following oral cancer surgery [24].
In addition to pain, one of the most suffering adverse events following bimaxillary orthognathic surgery is PONV. Although vomiting after orthognathic surgery is not directly life-threatening, it can cause dehydration, esophageal rupture, wound dehiscence, bleeding, hematoma, and aspiration of gastric contents, and in severe cases may lead to death [1]. Furthermore, PONV prolongs hospital stay, increases costs, and contributes to negative feelings regarding anesthesia and surgery [25]. It is known that the orthognathic surgery itself causes PONV to occur at a frequency as high as 30–50% [126]. Furthermore, use of opioids such as morphine after surgery may increase the incidence of PONV.
Various attempts have been made to reduce the incidence of PONV. Factors that influence the incidence of PONV include age, gender, obesity, vomiting history, psychological state, type and duration of surgery, and type of anesthesia. ppropriate use of antiemetics and analgesics with low risk of adverse effects is important.
Nefopam is a drug that induces central analgesia with an efficacy ratio of about 0.2 to 0.6: 1 (nefopam: morphine). The mechanism of nefopam-induced analgesia has not been clearly established. However, it may inhibit reuptake of neurotransmitters such as norepinephrine, serotonin, and dopamine, and may exert anti-hyperalgesic activity through inactivation of the N-methyl-D-aspartic acid receptor [272829]. Nefopam does not cause respiratory depression, does not inhibit the central nervous system, and has a low risk of nephrotoxicity, cardiovascular toxicity, and abuse [151630313233]. Moreover, nefopam has a definite advantage over NSAID in that it does not cause gastric mucosal erosion or affect platelet aggregation [30]. When used for control of postoperative pain, nefopam can reduce complications caused by opioids by reducing the amount of opioids used [3435]. Recent reports have suggested that NSAIDs can impede bone healing [1819], which is not a known side effect of nefopam, which adds to its benefit as a means for postoperative pain control following orthognathic surgery [20].
Although nefopam can induce adverse effects such as tachycardia, sweating, nausea, vomiting, drowsiness, asthenia, light headedness, and pain at the injection site, these symptoms are typically transient and non-severe [35363738]. Nefopam has recently been reported to lower PONV after laparoscopic gynecologic surgery and cardiac surgery [151617].
In this prospective, double-blind, randomized, and controlled study, we investigated the incidence of PONV, postoperative pain, and other adverse effects in patients who used nefopam or fentanyl for PCA after a bimaxillary orthognathic surgery.
In this study, the pain control effects of nefopam were comparable to those of fentanyl in patients who underwent bimaxillary orthognathic surgery, and there were no significant differences in the incidences of other adverse effects. However, nefopam did not lower PONV in our study.
However, other studies have reported that using nefopam in conjunction with opioids for PCA has opioid sparing effects, which can result in a lower incidence of PONV than when using opioids alone [1639404142]. We may not have observed any benefits of nefopam on incidence of PONV because our study population consisted of patients who underwent orthognathic surgery. A previous study also reported that there were no significant differences in postoperative pain control and incidence of PONV following administration of nefopam or fentanyl [43].
In our study, although incidence of PONV was similar between the groups, patient satisfaction was higher in the F group than in the N group at 24 and 48 hours after surgery. However, previous studies reported no significant differences in satisfaction between the two drugs [43], while other studies reported that patients who received nefopam via PCA were more satisfied [9343944]. Thus, additional studies are needed to further clarify these discrepancies.
This study had several limitations. First, we could not meet our planned sample size. However, we do not believe that having met the planned sample size would have led to different results, and terminating the study due to a higher incidence of PONV in the N group with 89 patients in the interim analysis was the correct ethical decision. Furthermore, based on these findings, we could conclude that the incidence of PONV did not differ between the nefopam and fentanyl groups. Second, we could not standardize the type of rescue analgesics used in the recovery room and in the ward after surgery, and various analgesics, such as fentanyl, pethidine, and ketorolac were used. Furthermore, we could not administer antiemetics in proportion to the actual intensity of nausea and vomiting.
In conclusion, using nefopam for PCA after bimaxillary orthognathic surgery did not significantly reduce the incidence of PONV compared to fentanyl. Furthermore, there were no significant differences in postoperative pain control between nefopam and fentanyl. Based on these results nefopam is a safe drug that can be used to control postoperative pain.
Notes
References
1. Silva AC, O'Ryan F, Poor DB. Postoperative nausea and vomiting (ponv) after orthognathic surgery: A retrospective study and literature review. J Oral Maxillofac Surg. 2006; 64:1385–1397. PMID: 16916674.

2. Frost CM, Frost DE. Nursing care of patients in intermaxillary fixation. Heart Lung. 1983; 12:524–528. PMID: 6554262.
3. Niederhagen B, Braumann B, Dierke-Dzierzon C, Albrecht S. [postoperative pain after interventions in the area of the mouth-jaw-face]. Mund Kiefer Gesichtschir. 1997; 1:229–234. PMID: 9410633.
4. Park S, Chi SI, Seo KS, Kim HJ. Circadian variation of iv pca use in patients after orthognathic surgery - a retrospective comparative study. J Dent Anesth Pain Med. 2015; 15:141–146. PMID: 28879271.

5. Aoki Y, Yoshida K, Nishizawa D, Kasai S, Ichinohe T, Ikeda K, et al. Factors that affect intravenous patient-controlled analgesia for postoperative pain following orthognathic surgery for mandibular prognathism. PLoS One. 2014; 9:e98548. PMID: 24893040.

6. Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993; 5:182–193. PMID: 8318237.

7. Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand. 2001; 45:795–804. PMID: 11472277.
8. George JA, Lin EE, Hanna MN, Murphy JD, Kumar K, Ko PS, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: A meta-analysis. J Opioid Manag. 2010; 6:47–54. PMID: 20297614.
9. Tramoni G, Viale JP, Cazals C, Bhageerutty K. Morphine-sparing effect of nefopam by continuous intravenous injection after abdominal surgery by laparotomy. Eur J Anaesthesiol. 2003; 20:990–992. PMID: 14690106.

10. Rohm KD, Riechmann J, Boldt J, Schuler S, Suttner SW, Piper SN. Physostigmine for the prevention of postanaesthetic shivering following general anaesthesia - a placebo-controlled comparison with nefopam. Anaesthesia. 2005; 60:433–438. PMID: 15819761.
11. Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth. 2012; 108:423–429. PMID: 22290456.

12. McLintock TT, Kenny GN, Howie JC, McArdle CS, Lawrie S, Aitken H. Assessment of the analgesic efficacy of nefopam hydrochloride after upper abdominal surgery: A study using patient controlled analgesia. Br J Surg. 1988; 75:779–781. PMID: 3167526.

13. Lee JH, Kim JH, Cheong YK. The analgesic effect of nefopam with fentanyl at the end of laparoscopic cholecystectomy. Korean J Pain. 2013; 26:361–367. PMID: 24156002.

14. Kim YA, Kweon TD, Kim M, Lee HI, Lee YJ, Lee KY. Comparison of meperidine and nefopam for prevention of shivering during spinal anesthesia. Korean J Anesthesiol. 2013; 64:229–233. PMID: 23560188.

15. Bhatt AM, Pleuvry BJ, Maddison SE. Respiratory and metabolic effects of oral nefopam in human volunteers. Br J Clin Pharmacol. 1981; 11:209–211. PMID: 6783057.

16. Kim K, Kim WJ, Choi DK, Lee YK, Choi IC, Sim JY. The analgesic efficacy and safety of nefopam in patient-controlled analgesia after cardiac surgery: A randomized, double-blind, prospective study. J Int Med Res. 2014; 42:684–692. PMID: 24691459.

17. Yoon JU, Byeon GJ, Cheon JH, Choi YM, Ri HS, Baik SW. Post-operative intravenous patient-controlled analgesic efficacy of morphine with ketorolac versus nefopam after laparoscopic gynecologic surgery: A randomized noninferiority trial. Korean J Anesthesiol. 2016; 69:161–166. PMID: 27066208.

18. Su B, O'Connor JP. Nsaid therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013; 115:892–899. PMID: 23869068.

19. Marquez-Lara A, Hutchinson ID, Nunez F Jr, Smith TL, Miller AN. Nonsteroidal anti-inflammatory drugs and bone-healing: A systematic review of research quality. JBJS Rev. 2016; 4.

20. Baht GS, Nadesan P, Silkstone D, Alman BA. Pharmacologically targeting beta-catenin for nf1 associated deficiencies in fracture repair. Bone. 2017; 98:31–36. PMID: 28254468.

22. Dawson R, von Fintel N, Nairn S. Sedation assessment using the ramsay scale. (cover story). Emergency Nurse. 2010; 18:18–20.
23. Precious DS, Multari J, Finley GA, McGrath P. A comparison of patient-controlled and fixed schedule analgesia after orthognathic surgery. J Oral Maxillofac Surg. 1997; 55:33–39. discussion 40. PMID: 8994466.

24. Shin TJ, Park Y, Seo K-S, Han HJ, Kim HJ. Surgical invasiveness is important for determining severity of postoperative pain after oral & maxillofacial surgery. J Korean Dent Soc Anesthesiol. 2011; 11:9–15.
25. Gold BS, Kitz DS, Lecky JH, Neuhaus JM. Unanticipated admission to the hospital following ambulatory surgery. JAMA. 1989; 262:3008–3010. PMID: 2810644.

26. Rodrigo C, Campbell R, Chow J, Tong A. The effect of a 4-mg preoperative intravenous dose of ondansetron in preventing nausea and vomiting after maxillofacial surgery. J Oral Maxillofac Surg. 1996; 54:1171–1175. PMID: 8859234.

27. Gray AM, Nevinson MJ, Sewell RD. The involvement of opioidergic and noradrenergic mechanisms in nefopam antinociception. Eur J Pharmacol. 1999; 365:149–157. PMID: 9988097.

28. Rosland JH, Hole K. The effect of nefopam and its enantiomers on the uptake of 5-hydroxytryptamine, noradrenaline and dopamine in crude rat brain synaptosomal preparations. J Pharm Pharmacol. 1990; 42:437–438. PMID: 1979627.

29. Fuller RW, Snoddy HD. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology. 1993; 32:995–999. PMID: 7507578.

30. Dordoni PL, Della Ventura M, Stefanelli A, Iannace E, Paparella P, Rocca B, et al. Effect of ketorolac, ketoprofen and nefopam on platelet function. Anaesthesia. 1994; 49:1046–1049. PMID: 7864317.

31. Michael R, Younan N, Aziz M, Mostafa N, Ghobriel A, Gintautas J. Effect of a non-opiate analgesic, nefopam hydrochloride, on stress gastric ulcer in rats. Proc West Pharmacol Soc. 2001; 44:109–111. PMID: 11793953.
32. Alfonsi P, Adam F, Passard A, Guignard B, Sessler DI, Chauvin M. Nefopam, a nonsedative benzoxazocine analgesic, selectively reduces the shivering threshold in unanesthetized subjects. Anesthesiology. 2004; 100:37–43. PMID: 14695722.

33. Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain. 2014; 27:103–111. PMID: 24748937.

34. Du Manoir B, Aubrun F, Langlois M, Le Guern ME, Alquier C, Chauvin M, et al. Randomized prospective study of the analgesic effect of nefopam after orthopaedic surgery. Br J Anaesth. 2003; 91:836–841. PMID: 14633755.
35. Mimoz O, Incagnoli P, Josse C, Gillon MC, Kuhlman L, Mirand A, et al. Analgesic efficacy and safety of nefopam vs. Propacetamol following hepatic resection. Anaesthesia. 2001; 56:520–525. PMID: 11412156.

36. Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005; 100:169–174. PMID: 15616073.

37. Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: A review of its pharmacological properties and therapeutic efficacy. Drugs. 1980; 19:249–267. PMID: 6991238.
38. Djerada Z, Fournet-Fayard A, Gozalo C, Lelarge C, Lamiable D, Millart H, et al. Population pharmacokinetics of nefopam in elderly, with or without renal impairment, and its link to treatment response. Br J Clin Pharmacol. 2014; 77:1027–1038. PMID: 24252055.

39. Evans MS, Lysakowski C, Tramer MR. Nefopam for the prevention of postoperative pain: Quantitative systematic review. Br J Anaesth. 2008; 101:610–617. PMID: 18796441.

40. Oh CS, Jung E, Lee SJ, Kim SH. Effect of nefopamversus fentanyl-based patient-controlled analgesia on postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery: A prospective double-blind randomized controlled trial. Curr Med Res Opin. 2015; 31:1599–1607. PMID: 26047392.
41. Jin HS, Kim YC, Yoo Y, Lee C, Cho CW, Kim WJ. Opioid sparing effect and safety of nefopam in patient controlled analgesia after laparotomy: A randomized, double blind study. J Int Med Res. 2016; 44:844–854. PMID: 27358262.

42. Kim SY, Huh KH, Roh YH, Oh YJ, Park J, Choi YS. Nefopam as an adjunct to intravenous patient-controlled analgesia after renal transplantation: A randomised trial. Acta Anaesthesiol Scand. 2015; 59:1068–1075. PMID: 25903742.

43. Moon JY, Choi SS, Lee SY, Lee MK, Kim JE, Lee JE, et al. The effect of nefopam on postoperative fentanyl consumption: A randomized, double-blind study. Korean J Pain. 2016; 29:110–118. PMID: 27103966.

44. Tirault M, Derrode N, Clevenot D, Rolland D, Fletcher D, Debaene B. The effect of nefopam on morphine overconsumption induced by large-dose remifentanil during propofol anesthesia for major abdominal surgery. Anesth Analg. 2006; 102:110–117. PMID: 16368814.

Fig. 1
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patients in this trial. Eighty-nine patients were randomly assigned to the Nefopam (n = 48) or Fentanyl (n = 41) groups. At 72 hours after the procedure, 31 and 34 patients remained in each arm, respectively.
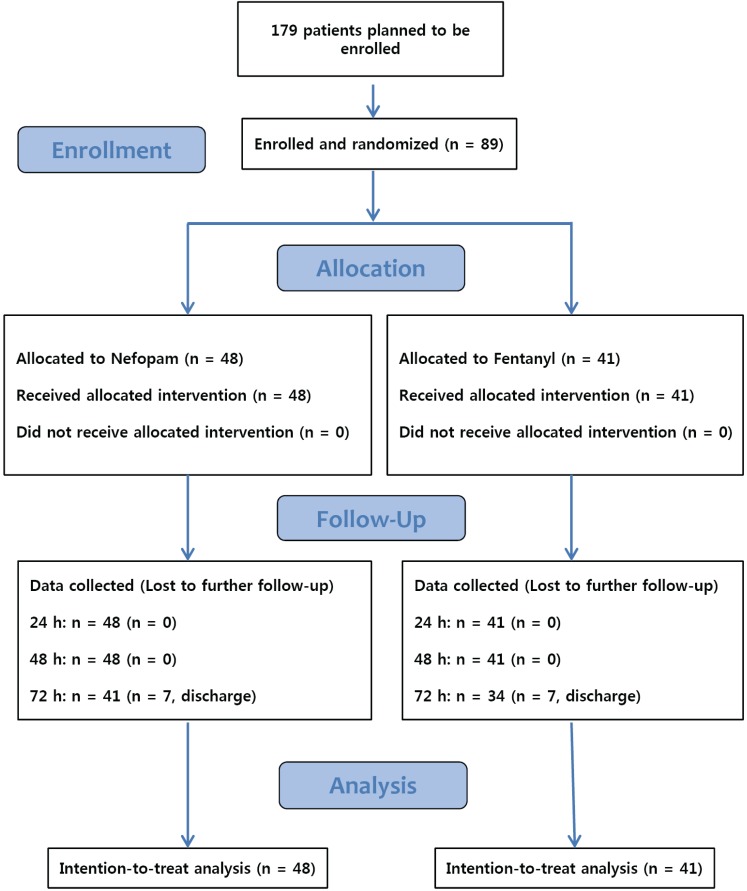
Table 1
Baseline demographic characteristics of study patients
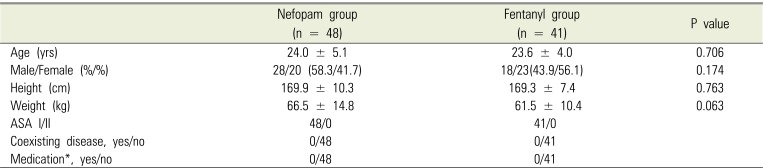
Table 2
Perioperative characteristics of study patients
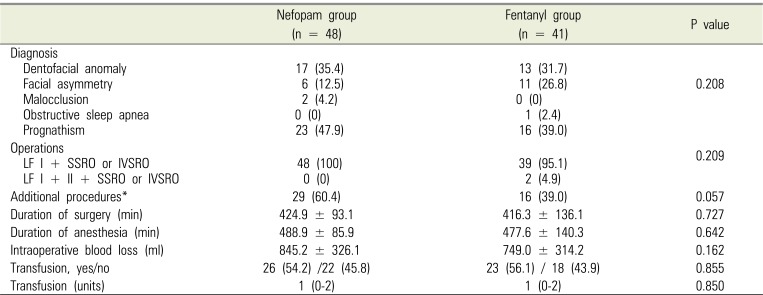
Values are presented as number (%), mean ± standard deviation, or median (interquartile range). *Genioplasty, rhinoplasty, angle reduction, cyst enucleation, surgical extraction, malar augmentation, or inferior terbunectomy.
LF = Le Fort, IVSRO = Intraoral Vertico-Sagittal Ramus Osteotomy, SSRO =Sagittal Split Ramus Osteotomy.
Table 3
Incidence of postoperative nausea and vomiting at different time
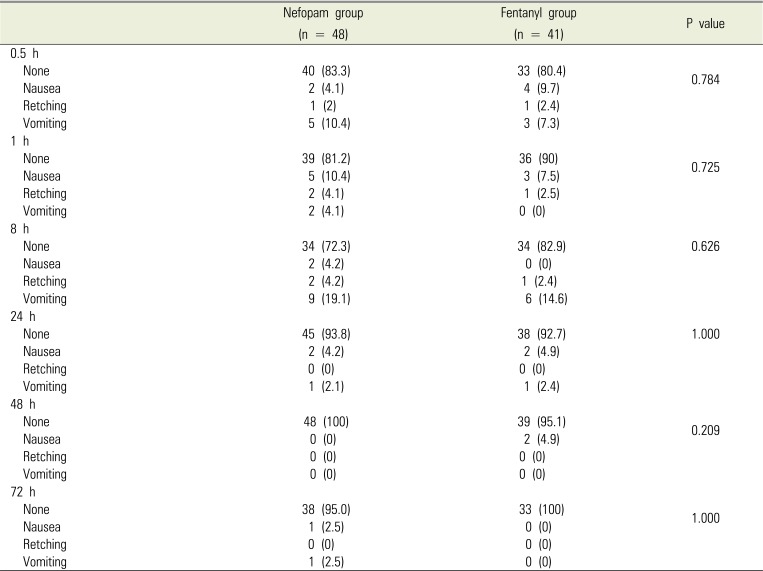
Table 4
Visual analogue scale (VAS) at different time
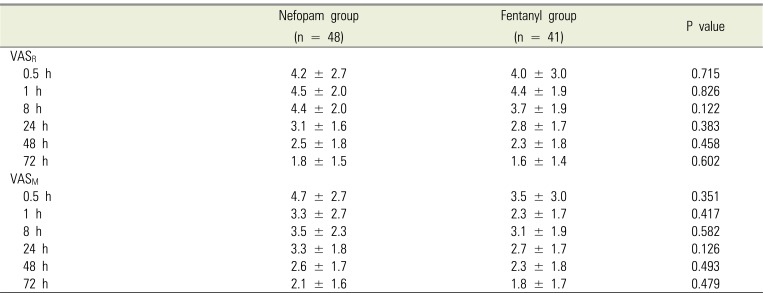
Table 5
Cumulative attempt and bolus delivery via patient-controlled analgesia device during 72 hr postoperatively
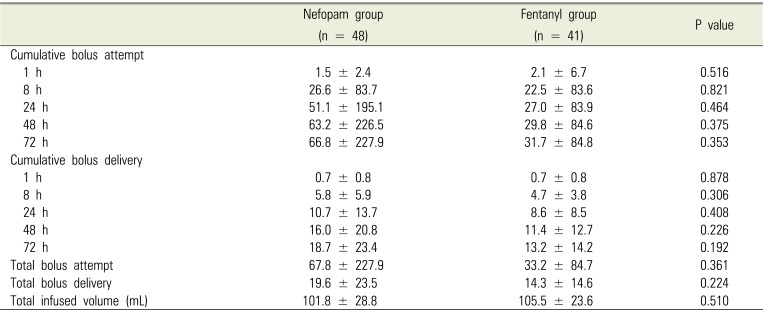
Table 6
Incidence of postoperative rescue analgesics and antiemetics at different time
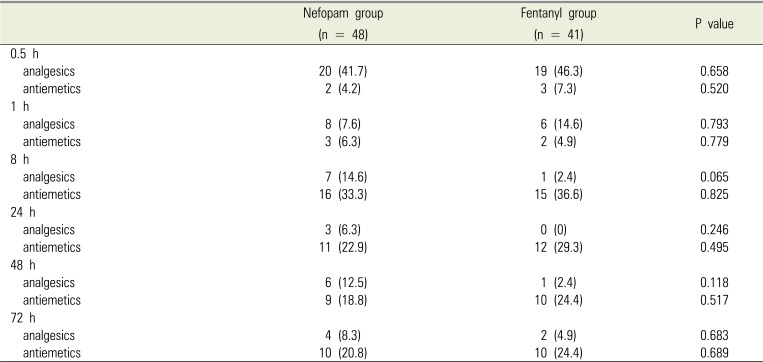
Table 7
Incidence of adverse events and patient satisfaction at different time following bimaxillary orthognathic surgery in patients receiving patient-controlled analgesia with nefopam or fentanyl




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download