Abstract
Malignant tumors that metastasize to the pancreas are rare. Among them, renal cell carcinoma is the most common. Surgical resection is more effective in treatment for patients with pancreatic metastasis from renal cell carcinoma, although targeted therapy is applied, to advanced renal cell carcinoma. It is essential to know exact medical history of the patient, because metastasis can occur late after nephrectomy. Surgical procedure may vary, depending on location and number of tumors. We report a case of resection of a pancreatic head tumor, 20 years after nephrectomy due to renal cell carcinoma.
Renal cell carcinoma (RCC), is the third most common malignant tumor in the kidney. Approximately 25% of RCCs are diagnosed with distant metastasis, and approximately 25% metastasize after nephrectomy. Median survival of metastatic RCC is 6 to 12 months, and 5-year survival, is less than 20%.1 Several retrospective comparative studies have reported, that resection of metastatic organs, prolongs overall survival.2 Malignant tumors arising in the pancreas are rare, but among them, RCC is reported as the most marked.3 Malignant tumors other than RCC, are considered to be widespread systemic diseases, when they metastasize to the pancreas. However, when RCCs metastasize to the pancreas, surgical resection as that for local disease, is considered. We report a case of pylorus-preserving pancreticoduodenectomy in a patient who underwent nephrectomy for RCC 20 years ago.
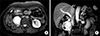
A 70-year-old female patient underwent right nephrectomy for RCC 20 years ago. In recent tests, two hypervascular masses of 2.2 cm and 5.0 cm in the pancreatic head, and right retroperitoneal space, were observed in abdominal computed tomography scan (Fig. 1) and magnetic resonance imaging (Fig. 2). Endoscopic ultrasonography-guided fine needle aspiration with a 22-G biopsy needle was performed twice, for histologic confirmation, but was not confirmed. Positron emission tomography-computed tomography was performed, to confirm distant metastasis, and mild fluorodeoxyglucose (FDG) uptake was shown at the tumor site (Fig. 3). Tumor markers such as carcinoembryonic antigen, cancer antigen 19-9, and alpha fetoprotein, were within normal range. Surgical resection was planned, and pylorus-preserving pancreaticoduodenectomy was performed (Fig. 4). There was mild adhesion, due to previous surgery, but surgery was performed without specificity. Histologic examination revealed metastatic RCC of clear cell type. She was discharged at postoperative day 12. The patient is currently undergoing outpatient follow-up, without recurrence for 10 months postoperatively.
Metastasis to the pancreas is rare, and accounts for approximately 2–5%.4 Among them, pancreatic metastasis from RCC is often common, and usually proceeds slowly, and exhibits indolent behavior. It may take 10 to 32 years to recur.4 Pancreatic metastasis in this case, also took 20 years after nephrectomy, to recur. Despite advances in medical technology, it is difficult to differentiate between primary carcinoma of the pancreas, and solitary pancreatic metastasis, so exact patient medical history is critical for diagnosis.
Somatostatin receptor scintigraphy, FDG-positron emission tomography, or endoscopic ultrasonography with biopsy could be performed; however, an accurate medical history is critical for diagnostic workup of such slow-growing tumor.5
Endoscopic ultrasonography-guided fine needle aspiration is considered the best method, and accuracy is reported to be more than 90% in the case of pancreatic adenocarcinoma.6 However, it is reasonable to perform surgery without fine needle aspiration biopsy, because pre-operative diagnosis could not change the treatment method in resectable tumors.
Tosoian et al.7 suggest that metastatic RCC in the pancreas is largely characterized by three presenting features: an extended disease-free interval after initial nephrectomy, with median interval ranging from 6 to 12 years; frequent discovery in the asymptomatic patient; and presence of isolated metastasis in the absence of widespread disease. These findings emphasize the importance of long-term follow-up, after initial nephrectomy. Also, data of previous studies showed the high rate of vascular invasion and rarity of lymphatic involvement, may further support predilection of RCC of metastasize via vasculature. Too, vascular invasion is the significant predictor of outcome.
Since Food and Drug Administration (FDA) approval of targeted therapies of metastatic RCC in 2005, systemic therapy for advanced RCC has changed dramatically.89 However, many of these drugs have improved overall survival, but the extent is minimal. Also, complete remission is rare, and there is slight chance of treatment. In addition, there are no data on usefulness of targeted therapy in cases of metastasis to the pancreas alone. In a recent study on surgical resections of pancreatic metastases from RCC, Schwartz et al. reported 3-, 5-, and 10-year overall survival of 72, 63, and 32%, respectively.10 Thus, targeted therapy is recommended, as treatment after surgical resection of metastatic RCC.
Among the case series in which RCC metastasized to the pancreas, 9 series with a case number of 7 or more, were investigated from 2010 (Table 1). In most studies, overall survival was more than 50% after pancreatic resection.
Konstantinidis et al.11 did not show prognostic factor such as disease-free interval, number of metastases, or tumor size.
RCC can metastasize to various organs such as the lung, adrenal gland, liver, pancreas, and thyroid gland. When metastatic tumors are surgically resected, 5-year recurrence-free survival differs slightly from each organ. Jakubowsk et al. reported a 5-year recurrence-free survival of 22% in the lung, 32% in the adrenal gland, 27% in the liver, and 43% in the pancreas.12 They also suggest that resection should be attempted at an organ site, if complete resection can be achieved regardless of length of the disease-free interval.
However, Wiltberger et al.13 suggested that multi-visceral resection should be considered, to achieve complete resection (R0) for good long-term outcomes, even though morbidity tended to be higher, than that with standard resection.
Benhaim et al.14 reported that radiofrequency ablation or cryoablative therapy could be performed in addition to surgery, even if the tumor is resectable. In fact, a patient who underwent cryoablative therapy, reported no complications or recurrence.
In the previously published clinical series, lymph node metastasis was rare in metastatic RCC of the pancreas. However, according to Schwarz et al., lymph node metastasis is common, and suggests the prognosis is worse.10 Thus, standard pancreatic resection with standard lymphadenectomy should be considered, rather than limited resection. In our case, there was no metastasis to the lymph node.
Rückert et al.15 reported that 3 of 40 patients developed post-operative pancreatic fistula grade C, postpancreatectomy hemorrhage grade B occurred in 6 patients, and 3 patients died after surgery. Mortality rate is considered to be somewhat higher. The authors believe the pancreatic tissue of the metastatic pancreatic cancer was so soft, that more pancreatic fistula or other complications developed. In addition, lymph node metastasis was found in 5 patients, and lymphadenectomy with pancreatectomy was recommended.
Tumors that have metastasized to the pancreas, may be solitary or multiple. Surgical methods are pylorus-preserving pancreaticoduodenectomy, distal pancreatectomy, or total pancreatectomy. It depends on the location and number of tumors.1316 In the case of solitary lesion, laparoscopic pancreatic resection may be performed.17 In fact, mortality rate from pancreatectomy has significantly decreased over the last 30 years, and resection of metastatic lesions improved quality of life and prognosis. Mortality rate of pancreatectomy from RCC metastasis has been reported at 0% to 6.4%.18 We need to make a careful decision regarding the surgical patient.
In conclusion, patients with pancreatic metastasis of RCC can expect long-term survival in surgical resection. Sometimes, it occurs late after nephrectomy (R0 resection), and a good prognosis can be expected in such cases. Although large-scale studies and randomized controlled trials, could not be performed because the patient population is small, surgery is considered a treatment of choice in these patients.
Figures and Tables
Fig. 1
Axial (A) and coronal (B) contrast-enhanced computed tomography scan images. Computed tomography scan shows a 5 cm well-defined hypervascular heterogeneous lesion and another 2.2 cm round enhancing mass in the right retroperitoneum along the 2nd duodenal portion. This mass involves the pancreatic head.
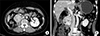
Fig. 2
T2-weighted fat-saturated magnetic resonance imaging finding. Two variable-size hypervascular mass lesions in the pancreatic head and right retroperitoneum suggested metastatic lymph node enlargement, from primary hypervascular mass lesion, such as renal cell and neuroendocrine carcinomas.
References
1. Flanigan RC, Campbell SC, Clark JI, Picken MM. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003; 4:385–390.


2. Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. 2008; 15:1161–1168.


3. Sweeney AD, Wu MF, Hilsenbeck SG, Brunicardi FC, Fisher WE. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2009; 156:189–198.


4. Ballarin R, Spaggiari M, Cautero N, De Ruvo N, Montalti R, Longo C, et al. Pancreatic metastases from renal cell carcinoma: the state of the art. World J Gastroenterol. 2011; 17:4747–4756.



5. Moletta L, Milanetto AC, Vincenzi V, Alaggio R, Pedrazzoli S, Pasquali C. Pancreatic secondary lesions from renal cell carcinoma. World J Surg. 2014; 38:3002–3006.


6. Hasan MK, Hawes RH. EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012; 22:155–167. vii


7. Tosoian JJ, Cameron JL, Allaf ME, Hruban RH, Nahime CB, Pawlik TM, et al. Resection of isolated renal cell carcinoma metastases of the pancreas: outcomes from the Johns Hopkins Hospital. J Gastrointest Surg. 2014; 18:542–548.


8. Conti SL, Thomas IC, Hagedorn JC, Chung BI, Chertow GM, Wagner TH, et al. Utilization of cytoreductive nephrectomy and patient survival in the targeted therapy era. Int J Cancer. 2014; 134:2245–2252.



9. Pal SK, Nelson RA, Vogelzang N. Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PLoS One. 2013; 8:e63341.

10. Schwarz L, Sauvanet A, Regenet N, Mabrut JY, Gigot JF, Housseau E, et al. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann Surg Oncol. 2014; 21:4007–4013.


11. Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C, et al. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010; 211:749–753.



12. Jakubowski CD, Vertosick EA, Untch BR, Sjoberg D, Wei E, Palmer FL, et al. Complete metastasectomy for renal cell carcinoma: comparison of five solid organ sites. J Surg Oncol. 2016; 114:375–379.



13. Wiltberger G, Bucher JN, Krenzien F, Benzing C, Atanasov G, Schmelzle M, et al. Extended resection in pancreatic metastases: feasibility, frequency, and long-term outcome: a retrospective analysis. BMC Surg. 2015; 15:126.



14. Benhaim R, Oussoultzoglou E, Saeedi Y, Mouracade P, Bachellier P, Lang H. Pancreatic metastasis from clear cell renal cell carcinoma: outcome of an aggressive approach. Urology. 2015; 85:135–140.


15. Rückert F, Distler M, Ollmann D, Lietzmann A, Birgin E, Teoule P, et al. Retrospective analysis of survival after resection of pancreatic renal cell carcinoma metastases. Int J Surg. 2016; 26:64–68.


16. Yagi T, Hashimoto D, Taki K, Yamamura K, Chikamoto A, Ohmuraya M, et al. Surgery for metastatic tumors of the pancreas. Surg Case Rep. 2017; 3:31.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download