IV. Discussion
Bioceramics is an advancing front in the reconstruction of defects of the maxillofacial skeleton. Various biomaterials have been evaluated as bone substitutes
123. The volume needed for the reconstruction of maxillofacial defects without causing secondary morbidity in a patient with an autogenous graft is difficult to acquire, so alternative bone substitute materials have been investigated for a long time
1. The cost involved for synthetic materials and autogenous grafting is not affordable to the common man, so the search for an economical ideal substitute material is ongoing
4. In the recent past, eggshell derivatives have been assessed as scaffold materials
11121314. More recently, an eggshell-derived bone graft substitute material was introduced and studied as a synthetic bone graft substitute in a few animal
151617 and human clinical studies
5612.
Eggshell has been used for various medical formulations
78. The eggshell contains calcium precursors, typically calcium carbonate (94%), calcium phosphate (1%), organic matter (4%), and magnesium carbonate (1%)
78. The weight percentages of the constituents are Ca (35.11%), P (3.66%), C (15.28%), O (44.59%), and Mg (1.37%)
7. Every day, a million tons of eggshells are produced as biological waste around the world
7. Various production technologies for the synthesis of HA from different raw materials have been tried with varying success. Important issues in the industrial process are the minimization of waste and ability to recycle waste materials into useful products
7. The synthesis of HA from chicken eggshell has been tried in the laboratory with different methods like hydrothermal, wet precipitation, sol-gel, and microwave irradiation processes
7. The nanotechnology-assisted production of HA as a bone substitute is a dream come true for the surgeons working on artificial bone regeneration
151617. The advantage of HA is its great compositional similarities to the main constituent of human bone and its ability to process osteoprogenitor cells
13. The complex yet highly effective intracellular signaling of osteogenesis will be triggered by the presence of soluble calcium and inorganic phosphates
13.
The ideal bone substitute should be biocompatible, osteo-inductive or at least osteo-conductive, and have satisfactory mechanical properties
11. This bone substitute should be available in unlimited quantities with low cost
11. To accomplish these goals, the use of synthetic bone substitutes has been increasingly considered
11. Eggshell powder has shown promising results in preliminary animal studies
11121314. The experiments with animals in four groups have shown satisfactory healing, but osteo-induction has not been observed
1112. Eggshell powder has demonstrated no toxicity in animal experiments
11121314. This natural product has already been used as a mineral additive in a chewing gum without adverse reaction; it is inexpensive and can be found in an unlimited quantity
11. Recently, the eggshell powder has been processed and converted into HA, which has been evaluated in different animal models with promising results
151617. EHA has been produced by our associates using a domestic microwave process and this environmentally friendly conversion technique of conversion has been patented
7. The preliminary studies have shown promising results in a small group of patients
56.
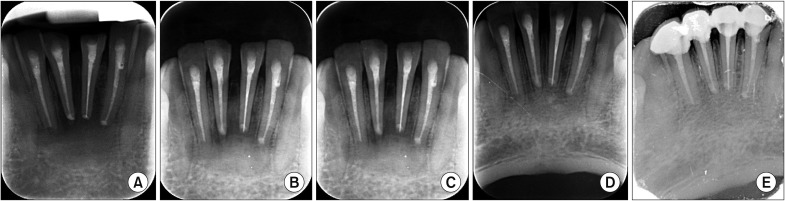
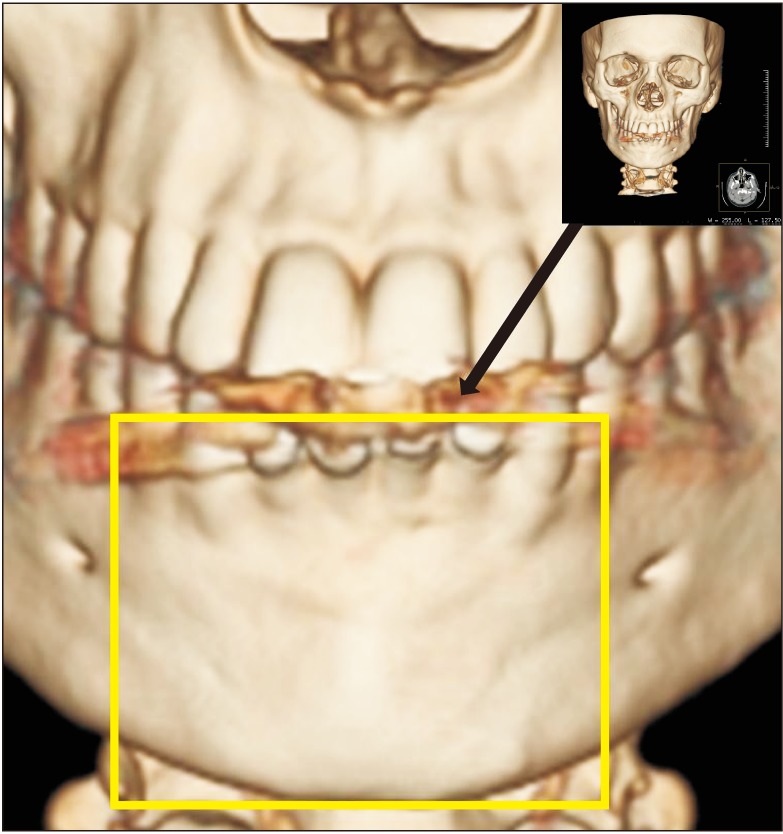
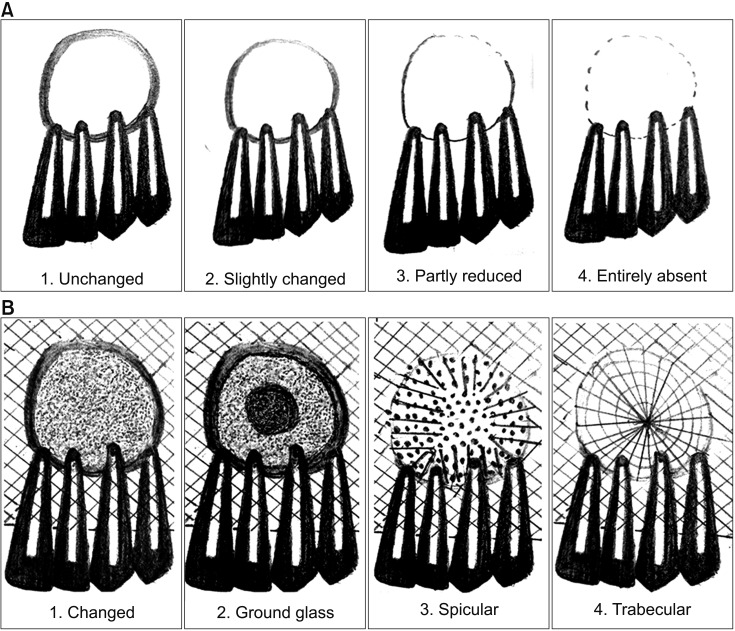
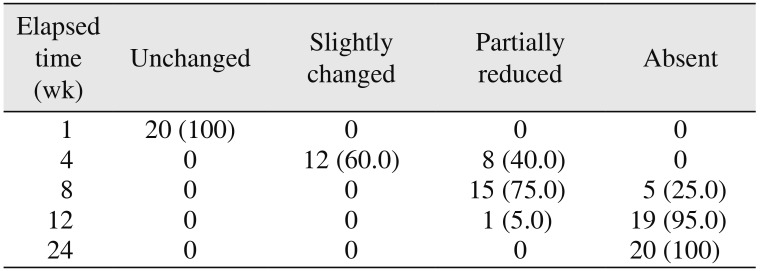
In the present study, the surgical site outline is the outline of the surgical defect after cystic enucleation, which will change with time because of creeping substitution from the periphery and will merge with the material margin in the due course of time
910. The surgical site outlines were categorized as unchanged (altered less than 25%), slightly changed (more than 25% and less than 75%), partially reduced (more than 75%), or absent (inability to differentiate between graft material outline and surgical site outline)
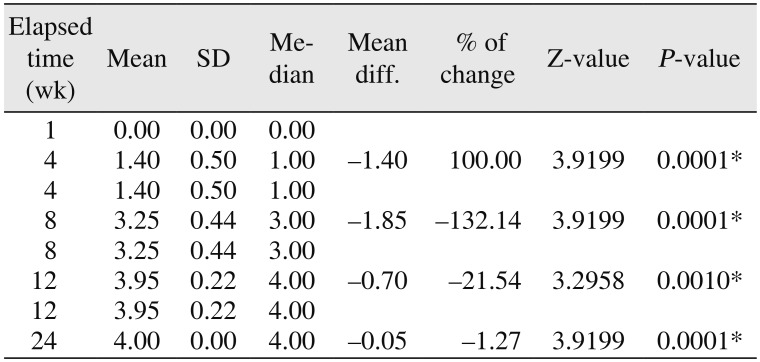
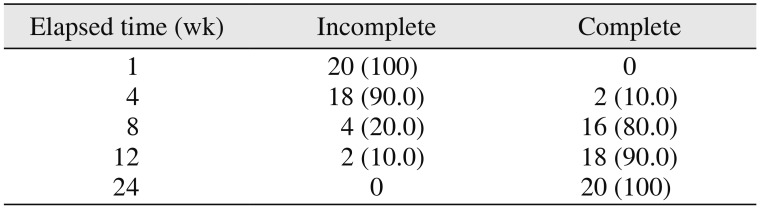
910. In the present study, the surgical site outline was slightly changed in 12 patients and partially reduced in eight patients by the fourth week and the eighth week, respectively. The surgical site margin visibility was further reduced to partially by the eighth week in 15 patients and was completely absent in five patients, indicating the material diffusion into the adjacent bone or the creeping of bony substitute from the periphery; this is similar to previous studies published in the literature
56. The transition from the slightly reduced category to the partially reduced category was seen in seven patients over an interval of four weeks (i.e., from the fourth week to the eighth week).(
Tables 2,
3) HA derived from eggshells showed more new bone formation in the early stages than did the scaffolds made from commercially available powders in animal studies
1617, and this is also evident in our radiographic observations of bone formation. Another animal study compared EHA and synthetic HA (SHA) using a rat model, which showed significantly higher bone regeneration in the EHA group than the unfilled control at eight weeks
17. These results support our study findings. The merging of the material margin and the bony margin is indicative of new bone formation, as a creeping bone substitute and margins were invisible by the twelfth week.(
Tables 2,
3)
The formation of bone and the blending of the bone margin with the material margin were well correlated with the density of bone formation in the previous study
6, which supports our study observations. The margin blend with commercially available materials like bovine and SHA in the previous studies are similar to those of the present study, indicating that EHA is equally efficient in the enhancement of bone regeneration
10. The rapid bone regeneration associated with grafting could be explained by the ability of the HA to enhance the selective adsorption of attachment proteins and growth factors that stimulate osteoblast adhesion and bone deposition
18. Another mechanism by which the graft could enhance bone formation is through ion release, as supported by the work of Matsuoka and associates
19.
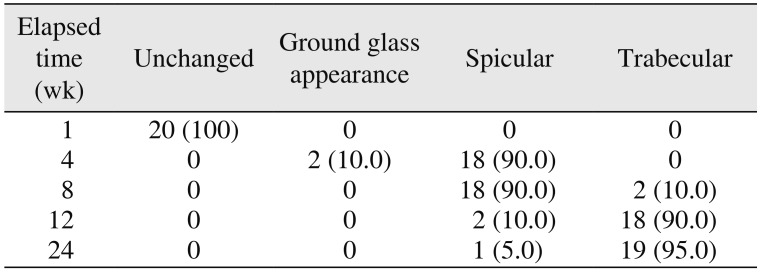
The bone formation characteristics are the radiological appearance of the surgical site as changed, ground glass, spiculed, or trabecular
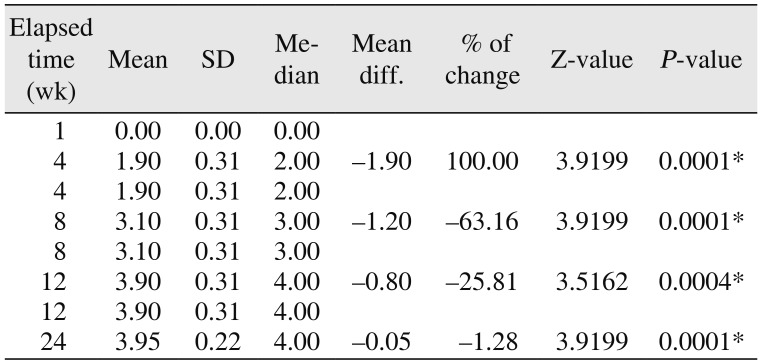
910. These radiological appearances depend on the visible characteristics of radiographs in terms of radio-opacity in comparison with the adjacent bone and trabecular pattern. The study showed 90% specular appearance at the eighth week postoperatively, indicating the new bone formation. The initial radio-opacity of the graft material was increased within four weeks, which is indicative of the osteoconductive nature of the material.(
Table 4) The study showed a trabecular pattern in 18 patients by the twelfth week, which indicated complete bone formation in 90% of the patients.(
Table 4) The bone formation was significant during the time intervals from the fourth to the eighth week and from the eighth to the twelfth week.(
Table 5) Kim et al.
16 showed that the bone formation around the HA scaffolds fabricated from eggshells was more active than that of the control groups in rabbits. Similarly, Lee et al.'s animal study
17 showed very similar results by the end of eight weeks, with 41.99%±8.44% of new bone formation in SHA, a significantly higher amount, when compared with the control. Small-sized particles have a larger surface area than large-sized particles with the same weight. Therefore, small-sized particles may be more easily removed from the body
17, as our HA is in the form of nanoparticles, which might be the reason for early bone regeneration. A microwave process with low temperature was used to prepare the HA in our study
7. The crystallinity of the EHA was higher than that of the SHA used in animal studies in the published literature
17. Highly crystalline HA substrate supports higher osteogenic cell proliferation than low-crystalline HA substrates
17. Most defect areas were filled with regenerated bone in the EHA group, and the remaining EHA particles were incorporated into the regenerated bone at eight weeks after the operation
17. This might be the reason for the complete trabecular patterns of radiographs in our study. The trabecular pattern was similar with the adjacent normal bone. Similar findings have been reported in an animal study by Kim et al.
16. The levels of new bone formation, lamellation, and maturation were greater in an EHA group compared to a synthetic group
16. In addition to these obvious advantages of the enhancement of bone regeneration
56, the results of these studies suggest that EHA have excellent bone formation ability
1617. EHA was associated with a significantly higher bone formation in this study also.
The bone densities of the grafted sites were examined using Digora (Soredex, Helsinki, Finland). Digital intraoral periapical radiographs acquired at 4, 8, 12, and 24 weeks post-surgery were tabulated for changes in density with reference to the initial densities immediately after grafting, which were considered the baseline reference densities. The changes in density were correlated with bone formation characteristics at relevant time intervals. These changes in density were due to new bone formation and graft material resorption
910. The marked density changes that were observed, indicating bone formation, are similar to results in the published literature
56. The trabecular pattern of bone formation had the highest density attained at 12 weeks of follow-up; these findings are supported by our previous studies
5610 and other published literature using HA-modified bioglass
18.
Calcium-deficient HA with surface microstructures is more favorable as a bone substitute compared to stoichiometric HA, because of its faster biodegradability and rapid formation of a surface apatite layer, enabling rapid bone-bonding microstructures
15. Our EHA is calcium deficient, which might be the reason for the enhancement of bone regeneration. Park et al.
15 showed that surface characteristics may contribute to the increased osteo-conduction of hydrothermally treated eggshells in the healing of rat calvarial defects, thus, the HA derived from chicken eggshell is an osteoconductive material for the enhancement of early bone regeneration
151617. Recent studies provided proof of concept for the clinical application of “smart” biomaterials, because the HA's osteo-inductive nature displayed superior biological performance through the modulation of cell behavior
202122. EHA-enhanced bone formation, and healing was complete by the end of 12 weeks.(
Table 6) This enhancement might be because of the calcium-deficient nature of EHA. The calcium-deficient characteristics of the surface HA layer in the new bone graft substitute (N-HA) derived from hen eggshell in Park et al.'s study
23 showed promotion of new bone formation by increasing protein adsorption and supplying sufficient Ca, which enhanced the reprecipitation and formation of calcium phosphate layers on its surface and subsequently influenced osteoblast responses. Even the histomorphometric observations revealed new bone formation and new bone islands with N-HA derived from hen eggshell prepared through hydrothermal processing, which is osteoconductive in nature
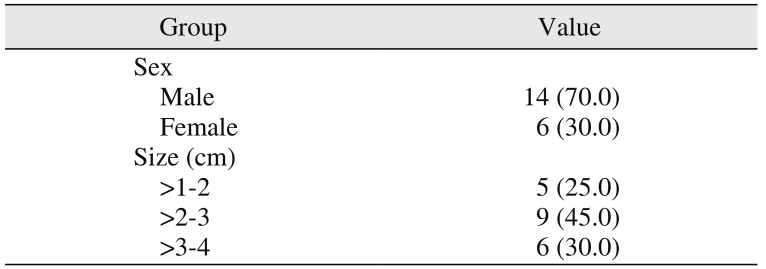
23. Our radiographic bone formation characteristics might be enhanced due to these above-mentioned facts at early stages within 8 to 12 weeks. The study samples were divided into three groups depending on the size of the lesions: >10–20 mm, >20–30 mm, and >30–40 mm.(
Table 1) However, the healing of bone was complete in all of the patients irrespective of the sizes of their lesions. The literature showed that tunnel lesions will heal with scarring or fibrous connective tissue if grafting is not attempted
2425. The published literature showed the necessity of grafting for early bone formation and enhancement, which supports our findings
232425. The majority of our cases were tunnel lesions; 45% of the patients had lesions >20–30 mm in size, and 30% had lesions >30–40 mm in size. In all cases, the healing was uneventful; 80% of patients showed complete bone healing by the eighth week, and 90% by the twelfth week. Within the group, the study didn't show any significant differences in the healing among all of the lesions. Regenerative therapy is essential for the enhancement of bone healing so that grafting prevents scar tissue formation
2425. The published literature supports the notion of grafting/regenerative techniques for early bone healing
26272829. However, none of the published studies used EHA. They used different grafting materials, including two studies that had control groups with Chitra granules
27 and anorganic bovine bone
28, and one study that used a freeze-dried bone allograft without a control group
29. All of these studies suggested that grafting or regenerative techniques enhance bone formation compared to control groups
2829.
In our study, wound dehiscence was seen in one case, which was attributed to improper surgical planning and an existing sinus within the flap. This case has been excluded from the evaluation due to seepage of graft material through the defect. None of the animal studies in the published literature showed any undue reactions to the EHA bone graft substitute
15161723. Eggshells are by products of food waste, so recycling them as a raw material for bone graft material production; is not only economical but also environment friendly because of ready availability of eggshell waste
1723. EHA is cost-effective and production is environment friendly with no disease transfer risks
56. This economical graft material can be used as raw material for the production of various shapes and sizes of grafts in various biomedical applications including three-dimensional construction
30.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download