Abstract
In Florida, where syphilis is a reportable disease, the number of primary and secondary (P&S) syphilis cases has increased from 3,266 in 2008–2010 to 5,340 in 2013–2015, a 63% increase. The objective of this study was to compare the performance and sensitivity of the syphilis reverse algorithm with the traditional algorithm for detecting P&S (infectious) syphilis cases. Clinical specimens from individuals who self-referred for syphilis testing at public health clinics were processed using the traditional algorithm (non-treponemal rapid plasma reagin (RPR) test followed by a confirmatory treponemal (EIA) test) and then further tested with the Architect Syphilis TP (ASTP) immunoassay (Abbott Diagnostics, Chicago, IL, USA) or by RPR confirmation, if needed (reverse algorithm). Of 1,079 specimens, 59 were positive for syphilis. The sensitivity of the reverse algorithm was 98.3% (58/59) and of the traditional algorithm was 72.9% (43/59). Based on clinical evidence, of the 16 traditional algorithm-negative but reverse algorithm-positive cases, 68.8% (11/16) were classified as missed P&S infections (treatment naïve) and 31.2% (5/16) were classified as missed past syphilis (latent or infections with documented linkage to care). The reverse algorithm enables the detection of additional P&S syphilis cases missed by our current traditional algorithm.
Syphilis is an ancient sexually transmitted disease (STD) caused by exposure to the bacterium Treponema pallidum. To date, syphilis remains a public health concern despite the availability of improved screening tests and effective and inexpensive antibiotic treatment. Early diagnosis of primary and secondary (P&S) syphilis cases is important because immediate treatment will prevent transmission and development of new cases. In Florida, where syphilis is a reportable disease, the number of P&S syphilis cases has increased by 63%, from 3,266 in 2008–2010 to 5,340 in 2013–2015 [1]. The diagnosis of syphilis is based on a combination of clinical history, risk assessment, symptom presentation, and serological test results [2]. A presumptive diagnosis of syphilis requires the use of two tests: a non-treponemal test (e.g., the rapid plasma reagin [RPR] test or the venereal disease research laboratory [VDRL] test) plus a treponemal test (e.g., enzyme immunoassay [EIA], chemiluminescence microparticle immunoassay [CMIA], or multiplex flow immunoassay [MFI]). Other acceptable treponemal tests include fluorescent treponemal antibody-absorbed or Treponema pallidum particle agglutination (TP-PA) [3].
The Florida Bureau of Public Health Laboratories (FBPHL) currently uses the traditional syphilis algorithm, in which a non-treponemal (RPR) screening test is followed by a confirmatory treponemal (EIA) test. This is currently the algorithm recommended by the U.S. Centers for Disease Control and Prevention (CDC), because non-treponemal tests are inexpensive, relatively easy to perform, and strongly correlated with disease status [4]. However, these tests are interpreted subjectively, require manual processing, and more importantly, are less sensitive than newer U.S. Food and Drug Administration (FDA)-approved treponemal screening alternatives [4]. Currently, a growing number of clinical and public health laboratories have adopted a reverse algorithm for syphilis testing, consisting of a treponemal test (most often EIA, CMIA, or MFI) followed by confirmation of screen-positive specimens with RPR. Discordant specimens that are positive according to the treponemal screening test but negative by RPR are then tested by a second, orthogonal treponemal test to confirm true-positive results.
The objective of this study was to compare the performance of the reverse algorithm (Abbott Architect Syphilis TP [ASTP] CMIA screen (Abbott Diagnostics, Chicago, IL, USA)/RPR confirmation/TP-PA if discordant) and the traditional algorithm (RPR screen/Trep-Sure Total EIA [Trinity Biotech, Jamestown, NY, USA] confirmation/TP-PA if discordant) and to determine which algorithm demonstrates greater sensitivity in detecting specifically P&S (infectious) syphilis cases in the FBPHL study population.
Between March 8, 2017, and April 5, 2017, the FBPHL, Jacksonville, retrospectively examined 1,079 well-characterized clinical serum specimens from individuals who self-referred to county public health clinics for syphilis testing. This study was exempted from approval by the Institutional Review Board of the Florida Department of Health, Tallahassee, FL, USA. All specimens were processed within 1–3 days post-collection (no freeze/thaw cycles), according to the traditional algorithm and then additionally tested using the ASTP CMIA. If the ASTP was reactive, the specimen was further tested according to the reverse algorithm. Positive syphilis cases were determined based on a serological profile of two concordant positive treponemal antibody tests or concordant positive treponemal and non-treponemal antibody tests [2]. To differentiate between past/latent or early infections, clinical and treatment data from the Florida Department of Health Patient Reporting Investigation Surveillance Manager (PRISM) database was reviewed. If PRISM did not indicate a latent, sero-fast, or treated status, a positive syphilis case was classified as a P&S infection. Algorithm sensitivity and specificity with respective 95% confidence intervals were calculated.
Of the 1,079 clinical specimens tested using the reverse and traditional algorithms, 59 were classified as positive for syphilis (infectious and non-infectious). The sensitivity of the reverse algorithm was greater than that of the traditional algorithm; however, the specificities of the two algorithms were similar (Table 1). Based on the PRISM database review, of the 16 traditional algorithm-negative but reverse algorithm-positive cases, 68.8% (11/16) were classified as missed P&S infections (treatment naïve), and 31.2% (5/16) were classified as missed past syphilis (latent or infections with documented linkage to care) infections (Fig. 1). Of the 42 specimens positive by both algorithms, 11 were determined to be P&S infections, and 31 were classified as non-infectious stages (early/late latent or treated infections).
In this study, the traditional algorithm failed to detect syphilis in 16 of 59 positive syphilis cases (27.1%), similar to a previous study of approximately 2,700 positive syphilis cases that had found a missed diagnosis rate of 24.2% [5]. Our comparison of the results of the reverse and traditional algorithms highlighted substantial differences in their abilities to detect P&S infections. In 2016, the Florida Department of Health STD Program reported 2,407 infectious cases of P&S syphilis, of which 20.6% (496/2,407) were reported by the FBPHL alone, using the traditional algorithm [6]. Had the FBPHL used the reverse algorithm, an additional 496 P&S cases might have been detected. This would change the state-wide estimate (based on the 2016 data) to a minimum of 2,903 (2,407+496) P&S cases per year.
Laboratories transitioning from the traditional to the reverse algorithm will experience several challenges, including an increase in testing cost. Earlier studies found that initial treponemal screening facilitates identification of probable latent infectious syphilis and its early diagnosis, thus warranting careful communication regarding serological interpretation [78]. The impact of increased laboratory costs and reassessing serological interpretations may be lessened through collaborations between prevention and patient care programs or shared service models between other public health laboratories. Moreover, the additional costs of reagents and automation associated with the reverse algorithm can be mitigated by cost savings due to averted downstream transmissions, as more P&S cases are detected and treated early. A 2008 study examining costs averted by STI prevention programs in the U.S. estimated the direct and indirect P&S treatment cost to be USD 572 and USD 112, respectively, or a total of USD 684 per person per year [9]. The early detection and prompt treatment of an additional 496 P&S cases, assuming a linear relationship of only one downstream transmission per case, would result in 496 averted cases. The total savings in treatment costs due to averted infectious syphilis cases can be conservatively estimated at USD 339,264 (496×684).
We focused on infectious syphilis detection and any determination of syphilis staging, previous testing, and treatment history was limited to data derived from the PRISM database. Sero-fast and reinfection cases are typically identified in PRISM; therefore, all newly reported cases were determined to be P&S infections. Cost analysis was limited to a conservative estimate based on treatment savings associated with averted subsequent transmissions due to P&S cases missed by the traditional algorithm. We do not intend to provide cost estimates for the two algorithms, as this is dependent on laboratory testing volume, possible need for automation, and whether testing can be integrated with another STD testing. Automation of the screening test could result in faster reporting (turn-around-time). Furthermore, we did not examine the sensitivity and specificity of all FDA-cleared automated TP immunoassays. We did not evaluate Trep-Sure or TP-PA as the primary screening test because they do not lend themselves to automation in our high-volume laboratory.
The reverse algorithm enables the detection of syphilis cases missed by our current traditional algorithm. The 63% state-wide increase in acute syphilis cases in 2008–2015 is highly concerning [1]. This increase in P&S syphilis, together with a three-year high HIV transmission rate (as of 2015), suggests a comorbidity and a synergistic relationship [110]. The common means of transmission for these two STDs and the clear evidence that syphilis infection facilitates HIV transmission is a public health concern [1011]. Additional cost data related to algorithm transition is needed to assess overall cost savings or burden to public health STD programs and laboratories, bearing in mind that the objective of testing is to enable early diagnosis of disease and facilitate timely access to medical care for infected persons and their sexual partners.
Acknowledgments
This study was made possible by the support of Dan Petkas, Abbott Diagnostics, Abbott Laboratories Inc., and Sally Fordan, Retrovirology Supervisor, Florida Bureau of Public Health Laboratories.
Notes
References
1. Florida Department of Health. Florida CHARTS. Updated on Jul 2018. http://www.floridahealth.gov/diseases-and-conditions/sexually-transmitted-diseases/std-statistics/index.html.
2. Association of Public Health Laboratories. Suggested reporting language for syphilis serology. Updated on Dec 2015. http://www.aphl.org/AboutAPHL/publications/ID_suggested_syphilis_reporting_lang_122015.pdf.
3. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Updated on Jul 2016. https://www.cdc.gov/std/tg2015/syphilis.htm.
4. Theel ES, Binnicker MJ. Reverse sequence screening for syphilis. Updated on Nov 2014. https://www.aacc.org/publications/cln/articles.
5. Tong ML, Lin LR, Liu LL, Zhang HL, Huang SJ, Chen YY, et al. Analysis of 3 algorithms for syphilis serodiagnosis and implications for clinical management. Clin Infect Dis. 2014; 58:1116–1124. PMID: 24550376.
6. Florida Department of Health STD and Viral Hepatitis Section, Bureau of Communicable Diseases. 2016 Reportable syphilis cases. Updated on Dec 2017. http://www.flhealthcharts.com/charts/otherindicators/nonvitalSTDdataviewer.aspx?cid=0144.
7. Mishra S, Boily M, Ng V, Gold WL, Okura T, Shaw M, et al. The laboratory impact of changing syphilis screening from the rapid plasma reagin to a treponemal enzyme immunoassay: a case-study from the Greater Toronto Area. Sex Transm Dis. 2011; 38:190–196. PMID: 20706176.
8. Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015; 22:137–147. PMID: 25428245.
9. Chesson HW, Collins D, Koski K. Formulas for estimating the costs averted by sexually transmitted infection (STI) prevention programs in the United States. Cost Eff Resour Alloc. Updated on May 2008. http://resource-allocation.biomedcentral.com/articles/10.1186/1478-7547-6-10.
10. Centers for Disease Control and Prevention, CDC. Fact sheet, reported STDs in the United States. Updated on Sep 2018. https://www.cdc.gov/std/stats.
11. CMiller BA, Hicks CB. Syphilis and HIV: intersection of two epidemics. Updated on Jul 2017. http://www.jwatch.org/ac201009030000001/2010/09/03/syphilis-and-hiv-intersection-two-epidemics.
Fig. 1
Reverse and traditional algorithm schematics with testing population distribution.
*Based on the PRISM review of the 42 positive syphilis cases common to both algorithms, only 11 were reported as P&S (infectious) cases; †Clinical evaluation should be performed to identify signs, symptoms, or history of infection; ‡Syphilis staging as per PRISM review.
Abbreviations: P&S, primary and secondary; RPR, rapid plasma reagin; ASTP, Abbott Architect Syphilis TP; TP-PA, Treponema pallidum particle agglutination; EIA, enzyme immunoassay; CMIA, chemiluminescence microparticle immunoassay.
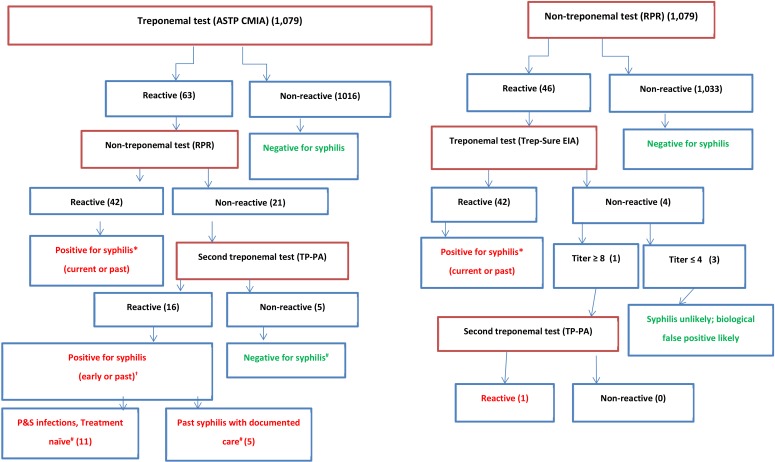
Table 1
Comparison of the reverse and traditional algorithms

*Of the 16 nonreactive RPR results reported as negative for syphilis per the traditional algorithm, 11 (68.8%) were classified as P&S infections and five (31.2%) as past syphilis (with documented linkage to care), using the reverse algorithm.
Abbreviations: P&S, primary and secondary; RPR, rapid plasma reagin; ASTP, Abbott Architect Syphilis TP; TP-PA, Treponema pallidum particle agglutination; EIA, enzyme immunoassay; CI, confidence interval.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download