Abstract
Purpose
The aim of our study was to prospectively evaluate the distribution of gamma-delta (γδ)1 and γδ2 T cells and their phenotypes in peripheral blood and prostate samples of patients diagnosed with or without prostate cancer (PCa) at prostate biopsy.
Materials and Methods
A consecutive series of 43 outpatients underwent trans-rectal echo-guided prostate biopsy for suspected PCa. Flow cytometry analysis was used to identify and characterize the γδ T cells populations in peripheral blood and tissue samples. Patients were stratified according to the presence or not of PCa, and its International Society of Urological Pathology (ISUP) grade (1 vs. ≥2).
Results
The distribution of γδ T cells in peripheral blood and prostate tissue showed wide variability and non-significant differences. A slightly higher percentage of δ2 T cells and a slightly lower percentage of δ1 T cells were found in peripheral blood of cancer patients. A non-significantly higher percentage of both Vδ1 and Vδ2 was expressed in cancer tissues, but a trend for lower distribution of δ1 and δ2 T cells was observed in ISUP grade ≥2. The “central memory” and “effector memory” were the most expressed T cells phenotype in peripheral blood and tissue samples. However no substantial differences in T cells subtypes distribution between cancer and healthy tissue were observed.
Conclusions
No substantially different percentages of γδ T cells were found in peripheral blood and biopsy samples of healthy and PCa patients. However a non-significant trend for lower infiltrate in higher ISUP grade cancer tissue was observed, suggesting a possible role for the immunosurveillance of PCa.
Prostate cancer (PCa) is the most common malignant neoplasm in male patients. PCa is a heterogeneous tumor with potentially long natural and clinical history, reflected in its common finding during autopsies carried out for other causes of death. In fact, many PCa are not expected to have clinically significant progression [12].
There is good evidence that cancer can naturally be controlled by the immune system. Mononuclear cells often infiltrate tumoral tissues and their presence were found to improve prognosis in patients with several tumors [3].
Gamma-delta (γδ) T lymphocytes are effector cells that may play a critical role in cancer immunosurveillance and recent clinical trials support their use as immunotherapic agents, either via the adoptive transfer of ex vivo expanded γδ T cells or following the activation of γδ T cells in vivo by compounds such as phosphoantigens or aminobisphosphonates [4]
Three main populations of γδ T cells are recognizable on the basis of δ chain expression, phenotypic and functional parameters. In fact the functional responses of γδ T cells can be stratified by the V region of the γδ T cell receptor (TCR). The γδ T cells expressing the Vδ1 TCR chain are mostly found in epithelial and mucosal tissues, contrasting tissutal damage, infection or transformation. Conversely, the γδ T cells expressing the Vδ2 TCR chain are the most common circulating γδ T lymphocytes [5], and can also act as antigen-presenting cells [67], activating CD4+ T cells. Finally the Vδ3 T cells, represent only the 0.2% of the circulating T cells [8].
Vδ1 T cells primarily reside within epithelial tissues (therefore also in the prostate) as first line agents of immunosurveillance, binding major histocompatibility complex (MHC) Class I-related ligands [9], that may act as tumor associated antigens. On the contrary more than the 90% of the circulating γδ T cells express the Vδ2 receptor [10]. The Vγ9Vδ2 displays a broad reactivity against stress-mediated metabolites produced by both microbial agents and tumors.
Moreover, γδ T lymphocytes can be categorized for their different surface marker expression and effector functions as naive (Tnaive), central memory (Tcm), effector memory (Tem) and terminally differentiated (Temra) cells [11]. The γδ T cells development is not always completed in the thymus, as many of them migrate to the peripheral tissues from the bone marrow with immediate effector function [12]. While Tnaive and Tcm cells home to lymph nodes without immediate effector functions, Tem and Temra cells instead migrate to inflammation sites, displaying immediate effector functions [11].
Considering PCa as a model for the antitumor properties of local Vδ1 T and migrated Vδ2 T cells, the aim of this study was to prospectively evaluate the frequency, the phenotype and the effector function of γδ1 and γδ2 T cells in prostate biopsy specimens and peripheral blood samples according to the diagnosis of PCa (vs. healthy patients) and its International Society of Urological Pathology (ISUP) grade.
A consecutive series of 43 outpatients who underwent trans-rectal echo-guided prostate biopsy for suspicious PCa were enrolled. A standard 12 core sextant biopsy of the peripheral zone was performed using a 16 G needle. The aim of the study was to assess the effect of the presence of PCa and its ISUP grade on the distribution of γδ T cells in prostate biopsy specimens and peripheral blood samples. For the aim of the study two additional cores (one left and one right) from the marginal zone of the prostate gland and a peripheral blood sample were taken from each patient. A written informed consent to participate was obtained from all the patients involved. According to Italian rules (art. 13, DLgs n. 196/03), this study did not require authorisation by the local ethical committee [13].
For tumor-infiltrating lymphocytes (TILs) analysis, the sample tissue of the two additional cores was obtained fresh and immediately transported to the laboratory in sterile saline solution for processing. Tissue was first minced into small pieces and digested with collagenase type IV, hyaluronidase and DNAase (Sigma, St. Louis, MO, USA) for one hour at 37℃. After digestion, the cells extracted were washed twice in RPMI 1640 medium.
Whole blood samples obtained from the same patients have been used for the comparative analysis between immunological status in peripheral blood and in the tumor or healthy tissue. The peripheral blood mononuclear cells (PBMCs) were separated from whole blood by density gradient centrifugation using Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). Cells viability was checked by Trypan blue assay at microscopy.
TILs and PBMCs were stained with the same monoclonal antibodies (mAbs), acquired and analyzed by flow cytometry on FACSCalibur and FACS CANTO II. The entire γδ T cell population was characterized using anti-CD3 peridinin chlorophyll protein (PerCp)-, anti-Vδ1 fluorescein isothiocyanate (FITC)-, anti-Vδ2 phycoerythrin (PE)-, anti-CD27 allophycocyanin (APC)- and anti-CD45RA PE-Cy7-conjugated mAbs (BD Biosciences, Mountain View, CA, USA). On the basis of surface marker expression, we distinguished CD45RA+CD27+ Tnaive, CD45RA−CD27+ Tcm, CD45RA−CD27− Tem, CD45RA+CD27− Temra cells, according to previously published methods [11]. Considering the high specificity of the markers used, no unspecific autofluorescent or compensation background was assessed.
The gating strategy consisted in the progressive measurement of total lymphocytes and specific cell types (Fig. 1). For every sample 100,000 nucleated cells were acquired and values were expressed as percentage of viable lymphocytes.
Patients were stratified according to the presence or not of PCa at sextant biopsy; the cancer patients were then substratified according to the ISUP grade (1 vs. ≥2). Healthy patients were considered those with normal histological findings or benign prostatic hyperplasia (BPH). The means of continuous variables and the distribution of nominal variables were compared with Student's t-test and Pearson's chi-squared test, respectively. All statistical analyses were performed using MedCalc v12.0 (MedCalc Software bvba, Ostend, Belgium) and GraphPad Prism v7.0 (GraphPad Software Inc., La Jolla, CA, USA). A p-value <0.05 was considered statistically significant.
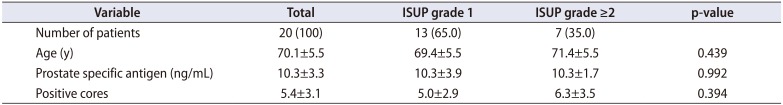
Overall, 43 patients were enrolled. After prostate biopsy 21 patients were diagnosed with cancer, 18 patients without cancer (healthy: normal or BPH) and 4 patients had prostatic intraepithelial neoplasia (PIN). For the aim of the study the patients with PIN were excluded. Three patients, one with and two without cancer were excluded because of the lack of data. Thus 36 patients, 20 PCa and 16 healthy patients were included in the analysis. Overall patients' characteristics are shown in Table 1. Within cancer patients, 13 (65.0%) and 7 (35.0%) men had ISUP grade 1 and ≥2, respectively (Table 2).
The γδ T cells analysis showed wide variability both on prostate biopsy and PBMC samples. Data showed a slightly higher percentage of δ2 T cells (2.6% vs. 2.1%) and a slightly lower percentage of δ1 T cells (0.5% vs. 0.7%) in peripheral blood of cancer patients in comparison with healthy patients, although not significant (Fig. 2A). Moreover the analysis of the PBMC samples showed a non-significantly higher percentage of Tem and Temra phenotypes in PCa patients (Fig. 2B).
On the contrary, TILs analysis showed a non-significant trend for higher expression of both δ1 and δ2 T cells in PCa patients (6.1% and 8.5% vs. 5.7% and 4.3% in healthy tissues, respectively) (Fig. 2C).
However, δ2 T cells obtained from peripheral blood of cancer subjects showed a predominant Tcm phenotype (38.5%), while the dominant phenotype in the PCa tissue was the Tem phenotype (40.3%). These results, although not statistically significant, could suggest that the cells committed to effector activities at the tumor site are phenotypically different respect to circulating δ2 T cell. Nevertheless the phenotypes of δ2 T cells in healthy and PCa tissue were substantially similar.
Regarding the δ1 T cells, the most prevalent PBMCs phenotype in PCa patients was Temra (40.7%), while the most prevalent tissutal phenotype was Tem (38.3%; Fig. 2B–D). On the contrary the most prevalent phenotypes in PBMCs of healthy subject were Tnaive and Tcm. However, also for the Vδ1 T cells, the distribution of the phenotypes in healthy and PCa tissue was substantially similar (not statistically significant).
The percentage of tumor-infiltrating γδ T cells according to ISUP grade is shown in the Fig. 3. A non-significant trend was found for lower percentage of infiltrating δ1 and δ2 T cell in more aggressive cancer (ISUP grade ≥2) in comparison with ISUP grade 1 (p=0.495 and 0.401, respectively). However, within the T cells subsets, the δ2 Tnaive were significantly more present in the high grade tumors (p=0.031; Fig. 3B).
In this study the circulating and tumor infiltrating γδ T cells were investigated in patients diagnosed with and without PCa at prostate biopsy. No substantially different percentages of γδ T cells were found in peripheral blood and biopsy samples of healthy and PCa patients. A non-significant trend for higher expression of both δ1 and δ2 T cells in cancer tissues and for lower expression in more aggressive cancer (ISUP grade ≥2) were found in comparison with healthy tissue and ISUP grade 1 cancer, respectively. Moreover we observed a slightly, although not significant, higher percentage of δ2 T cells and lower percentage of δ1 T cells in peripheral blood of cancer patients.
Although the effector functions of aß and γδ T cells are similar, γδ TCR binds also tumor-associated ligands in a MHC-independent manner [14], but the exact role of γδ T cells is controversial and not well understood yet. Specifically mediating the antigen-specific killing of cancer cells, γδ T cells have been associated with improved prognosis in patients with different types of carcinoma, in a retrospective analysis by Gentles et al. [15]. On the contrary some γδ T cells subgroups can activate immunosuppressive pathways and angiogenesis, supporting cancer progression. In this case overriding the interferon γ mediated antitumor immune response, interleukin (IL)-17 is an important mediator of such protumor functions [9]. IL-17 expression is higher in several human tumors, such as ovarian, cervical cancer, breast cancer, hepatocellular carcinoma, esophageal cancer, gastric and colorectal cancer [1416]. Interestingly, γδ T cells are rare and not recruited nor expanded within renal cell carcinomas and do not correlate with prognostic features nor specific death [17]. On the other hand, intratumoral γδ T cells amount was reported to be positively correlated with advanced stages and lymph node metastasis, and thus inversely correlated with both relapse-free and overall survival in breast cancer patients [18]. It is important to note that γδ T cells secrete IL-10 [19] and IL-17 [2021] regulating CD8+ T cell, neutrophils and monocytes recruitment and expansion, initiating the inflammatory response. Such functions could be exploited for immunotherapy; in fact promising results were reported in small-scale applications of Vγ9Vδ2 T cells to hematologic [22] and solid-tissue malignancies, including PCa and advanced renal cell carcinoma [23]. Moreover recent clinical trials support the use of γδ T lymphocytes as immunotherapeutic agents, either via the adoptive transfer of ex vivo expanded γδ T cells or following their activation in vivo [24].
The adult prostate contains several endogenous inflammatory infiltrates consisting of a variable amount of T and B lymphocytes, macrophages and mast cells [3], that may have an important role in cancer pathogenesis (progression or inhibition). T lymphocytes are often induced in PCa patients and infiltrate the tumor. In fact CD3+ and CD4+ T lymphocytes infiltrates have been shown to be clustered around PCa islets with only few lymphocytes scattered within the tumor area. CD3+ lymphocytes clusters contain numerous CD25+ and FOXP3+ cells with regulatory function, inhibiting immune response against cancer cells [25]. Also the CD8+ T cells are recruited into the prostate; however they express high levels of the inhibitory receptor PD-1, indicating exhaustion and inability of mounting an effective immune response [26].
Miller et al. [27] showed that CD4+CD25+ regulatory T cells (Treg) are increased in the tumor tissue and peripheral blood of early-stage PCa patients who had undergone radical prostatectomy compared with benign tissue from the same prostate and peripheral blood of normal donors, concluding that these cells may play a role in modulation of effector T cell responses against PCa.
Our study demonstrates a non-significant trend for higher expression of both δ1 and δ2 T cells in PCa tissue; the majority of PCa-infiltrating Vδ2 T cells showed Tem and Temra phenotypes, indicating cells homing to inflammation sites with immediate effector function as cytokine production. Their presence in the context of PCa could suggest a migratory process from the blood or lymphoid tissues, having a role in the immunosurveillance of this tumor. However also the γδ T cells found in healthy tissues primarily showed Tem phenotype. Conversely, circulating Vδ2 T from the same patients primarily showed Tcm phenotype that identifies cells homing to secondary lymphoid organs, without immediate effector function. Accordingly Norström et al. [28] reported a greatly different subset composition within lymphocytes isolated from BPH tissue sampled after trans urethral resection compared to those from peripheral blood, reflecting a different role of the immune system in different bodily environments. In particular both co-inhibitory and co-stimulatory receptors expressing T cells were described to be more present in BPH tissue compared to PBMCs, suggesting chronic activation and possible functional exhaustion of TILs. Moreover a recent study reported that the majority of T-cells infiltrating the prostate showed Tem phenotype and those from PCa tissue could be on average 20-fold higher than that obtained from controls (BPH and normal prostates); on the other hand Treg cells did not seem to be unique to the PCa setting, but they were frequently found in the prostate gland compared to peripheral blood regardless of prostate condition, again indicating a role in prostate homeostasis [29].
Finally it is noteworthy that in our population lower, although not significant, levels of infiltrating γδ T cells were found in more aggressive PCa (ISUP grade ≥2). Similarly, in a previous study reporting a higher percentage of Treg and T helper (Th) 17 CD4+ TILs in PCa tissue, a different T cells phenotype distribution was associated with PCa grade; in particular a preponderant Th17-mediated inflammation was associated with lower Gleason score [30].
If confirmed in larger studies, this could be important as the Gleason score is an independent prognostic factor of PCa and in many cases can guide the therapeutic choice (surveillance vs. active treatment) [1]
This small prospective study still cannot give answers on the potential independent prognostic value of the γδ T cells isolation on PCa bioptic specimens. One limitation could be that the TILs analyses were performed on two additional biopsy cores, without the certainty that the tissues sampled would be the same of the diagnostic ones (12 cores sextant biopsy). However it is known that the prostate tissue is highly variable and PCa is typically multifocal, unilateral or bilateral. According to this and the typical distribution of the TILs infiltrate already described in literature [25], we considered appropriate the analysis of the additional cores almost near to the positive ones. Moreover we considered as healthy those patients without cancer at biopsy, including those with normal histological findings and BPH; all the patients presented focal signs of inflammatory response at pathological examination, as usual in clinical practice.
This is the first report of the γδ T cells isolation on prostate biopsy specimen in relation to PCa diagnosis and ISUP grade in a cohort of subjects with homogenous clinical characteristic (age and prostate specific antigen values). Future studies should focus on the role of γδ T cells in PCa progression, but also in pre-malignant conditions as PIN.
The study of tumor infiltrating lymphocytes subpopulation is feasible in prostate biopsy samples. No substantially different percentages of γδ T cells were found in peripheral blood and biopsy samples of healthy and PCa patients. However a non-significant trend for lower infiltrate in higher grade cancer (ISUP grade ≥2) was observed. γδ T cells might have a central role in this context but we are still far from their clinical use. In fact the exact role of T cells in immunosurveillance and in tumor progression, particularly in relation to specific cytokines production, needs better understanding.
ACKNOWLEDGMENTS
Dr. Daniela Coniglio received a grant from the University of Palermo for her international Immunopharmacology PhD project entitled “Characterization of Human γδ T Cells Infiltrating Prostate Cancer.”
References
1. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005; 293:2095–2101. PMID: 15870412.

2. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–224. PMID: 12824459.

3. De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007; 7:256–269. PMID: 17384581.

4. Hao J, Wu X, Xia S, Li Z, Wen T, Zhao N, et al. Current progress in γδ T-cell biology. Cell Mol Immunol. 2010; 7:409–413. PMID: 21042298.

5. Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003; 102:2310–2311. PMID: 12959943.
6. Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. 2011; 68:2443–2452. PMID: 21573785.

7. Tyler CJ, McCarthy NE, Lindsay JO, Stagg AJ, Moser B, Eberl M. Antigen-presenting human γδ T cells promote intestinal CD4+ T cell expression of IL-22 and mucosal release of calprotectin. J Immunol. 2017; 198:3417–3425. PMID: 28330898.
8. Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J Immunol. 2013; 191:30–34. PMID: 23740951.

9. Rei M, Pennington DJ, Silva-Santos B. The emerging protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res. 2015; 75:798–802. PMID: 25660949.

10. Urban EM, Chapoval AI, Pauza CD. Repertoire development and the control of cytotoxic/effector function in human gammadelta T cells. Clin Dev Immunol. 2010; 2010:732893. PMID: 20396597.
11. Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003; 198:391–397. PMID: 12900516.
12. Jensen KD, Chien YH. Thymic maturation determines gammadelta T cell function, but not their antigen specificities. Curr Opin Immunol. 2009; 21:140–145. PMID: 19321327.
13. Tortorici S, Argo A, Buzzanca ML, Burruano F, Tetè S. Information, consent and therapeutic alliance in ambulatorial oral surgery. Ital Oral Surg. 2009; 8:155–164.
14. Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR, et al. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014; 111:E3562–E3570. PMID: 25114209.

15. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015; 21:938–945. PMID: 26193342.

16. Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014; 74:1969–1982. PMID: 24525743.

17. Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, et al. Questionable relevance of gamma delta T lymphocytes in renal cell carcinoma. J Immunol. 2008; 180:3578–3584. PMID: 18292585.
18. Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012; 189:5029–5036. PMID: 23034170.

19. Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. Eur J Immunol. 2008; 38:2274–2283. PMID: 18624301.
20. Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008; 29:90–100. PMID: 18585064.
21. Wu X, Zhang JY, Huang A, Li YY, Zhang S, Wei J, et al. Decreased Vδ2 γδ T cells associated with liver damage by regulation of Th17 response in patients with chronic hepatitis B. J Infect Dis. 2013; 208:1294–1304. PMID: 23847059.

22. Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003; 102:200–206. PMID: 12623838.
23. Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007; 56:469–476. PMID: 16850345.

24. Guo RT, Cao R, Liang PH, Ko TP, Chang TH, Hudock MP, et al. Bisphosphonates target multiple sites in both cis- and transprenyltransferases. Proc Natl Acad Sci U S A. 2007; 104:10022–10027. PMID: 17535895.

25. Ebelt K, Babaryka G, Frankenberger B, Stief CG, Eisenmenger W, Kirchner T, et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer. 2009; 45:1664–1672. PMID: 19318244.

26. Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate. 2009; 69:1694–1703. PMID: 19670224.

27. Miller AM, Lundberg K, Ozenci V, Banham AH, Hellström M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006; 177:7398–7405. PMID: 17082659.

28. Norström MM, Rådestad E, Sundberg B, Mattsson J, Henningsohn L, Levitsky V, et al. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget. 2016; 7:23581–23593. PMID: 26993768.

29. Rådestad E, Egevad L, Jorns C, Mattsson J, Sundberg B, Nava S, et al. Characterization of infiltrating lymphocytes in human benign and malignant prostate tissue. Oncotarget. 2017; 8:60257–60269. PMID: 28947968.

30. Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008; 14:3254–3261. PMID: 18519750.

Fig. 1
Gating strategy for flow cytometric analysis of lymphocytes. SSC-A, side scatter area; FSC-A, forward scatter area; FSC-H, forward scatter height; APC, allophycocyanin; PerCp, peridinin chlorophyll protein; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
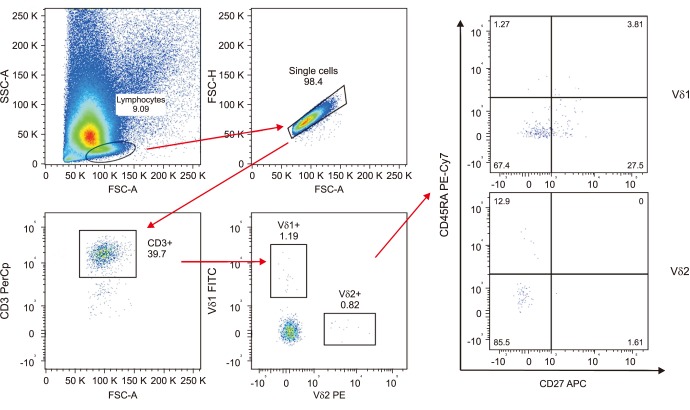
Fig. 2
(A) Percentage of total Vδ1 and Vδ2 T cells in peripheral blood samples of healthy and prostate cancer (PCa) patients. (B) Distribution of Vδ1 and Vδ2 T cells subsets in peripheral blood samples of healthy and PCa patients. (C) Percentage of total Vδ1 and Vδ2 T cells in prostate biopsy samples of healthy and PCa patients. (D) Distribution of Vδ1 and Vδ2 T cells subsets in prostate biopsy samples of healthy and PCa patients. Light blue columns identify Vδ1 and pink columns identify Vδ2 T cells. Tnaive, naive T cells; Tcm, central memory T cells; Tem, effector memory T cells; Temra, terminally differentiated T cells. Means were compared through Student t-test: p-values are reported above the corresponding columns.

Fig. 3
(A) Percentage of total Vδ1 and Vδ2 T cells in prostate biopsy samples of prostate cancer (PCa) patients stratified according to ISUP grade (1 vs. ≥2). (B) Distribution of Vδ1 and Vδ2 T cells subsets in in prostate biopsy samples of PCa patients stratified according to ISUP grade (1 vs. ≥2). Light blue columns identify ISUP grade 1 and pink columns identify ISUP grade ≥2. Tnaive, naive T cells; Tcm, central memory T cells; Tem, effector memory T cells; Temra, terminally differentiated T cells. Means were compared through Student t-test: p-values are reported above the corresponding columns (*p<0.05).
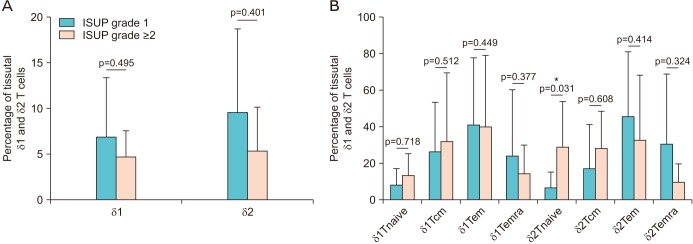
Table 1
Overall patients' characteristics
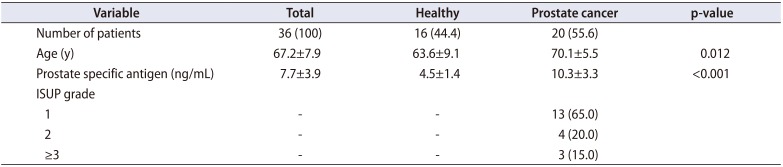
Table 2
Cancer patients' characteristics stratified according ISUP grade




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download