Abstract
Purpose
The aim of the study was to investigate the common bacteria found in the smegma in the subpreputial space of asymptomatic boys prospectively, and to determine the difference of those bacteria according to the presence of smegma.
Materials and Methods
In our institution, 40 boys who performed penoplasty were recruited into the study. Swab was done using aseptic techniques on smegma and glans in the operation room. According to the presence of smegma in the subpreputial space, we classified glans as a group S (with smegma, n=20) and group C (without smegma, n=20). The swabs were immediately sent to microbiology laboratory for microscopy, culture, and sensitivity tests.
Results
The mean age was 30.4±26.4 months. Thirty-one bacteria were isolated from smegma, comprising 15 gram-positive species (48.4%) and 16 gram-negative species (51.6%). The most commonly isolated gram-negative bacterium was Escherichia coli (25.8%), while the commonly isolated gram-positive bacteria were Enterococcus faecalis (19.4%) and Enterococcus avium (12.9%). Most of the bacterial isolates were multi-drug-resistant (61.3%). In group S, 12 boys had 22 bacterial isolates in the glans. The commonly isolated bacteria were E. coli (27.3%), E. avium (22.7%) and E. faecalis (18.2%). In group C, 13 boys had 21 bacterial isolates in the glans. The most commonly isolated bacterium was E. faecalis (28.6%).
An uncircumcised prepuce may be more readily colonized by bacteria and may cause more frequent urinary tract infections (UTIs) in children. In one study, 95% of infants with UTIs were uncircumcised [1]. In another study, among 5,261 infants, 41 (0.78%) had confirmed UTI in the first year of life. UTI occurred in only 0.47% of females and 0.21% of circumcised male infants, while it occurred in 4.12% of uncircumcised male infants [2]. Therefore, the incidence of UTIs is generally higher in the uncircumcised than in the circumcised, varying from 5 to 89 times [3].
The presence of an unretractable prepuce may facilitate colonization by uropathogenic bacteria and promote the adherence of bacteria, which may be important in the pathogenesis of UTIs [14]. Periurethral colonization may be an important initial step towards an ascending UTI [5]. The main pathogen of UTI is Escherichia coli in boys and girls [6]. E. coli with fimbriae (pili) will bind to the mucosal surface of the prepuce, but not to the outer skin surface [7].
Smegma is the secretion of the sebaceous gland that collects between the glans penis and the foreskin. The cheese-like sebaceous matter is a combination of shed skin cells, skin oils, and moisture. The main function of smegma is moisturing and lubricating the cavity between the foreskin and the glans that is the subpreputial space. [89].
Some studies showed that a variety of organisms was colonized in the subpreputial space [101112]. However, there was a controversy whether the smegma is the risk factor of UTI. Moreover, there was no study about the smegma that had never been exposed to the outside in the subpreputial space between the inner prepuce skin and glans surface before prepuce excoriate.
We hypothesis that the smegma that had never been exposed to the outside in the subpreputial space before prepuce excoriate is sterile. The aim of the study was to investigate the common bacteria with their antimicrobial susceptibility pattern found in the smegma in the closed subpreputial space of asymptomatic uncircumcised boys, and to determine the difference of those bacteria according to the presence of smegma prospectively.
This was a prospective study carried out at our hospital between August and December 2014. The study was approved by the ethics committee at the Pusan National University Yangsan Hospital (approval number: 05-2014-055).
Forty boys who presented of concealed penis in one institution during the period were enrolled. All the patient had the attached subpreputial space between the inner prepuce skin and glans surface.
We excluded the patient with UTI including balanoposthitis, past history of UTI, Kayaba's classification type III or IV of prepuce [13], and recent use of an antimicrobial or immunosuppressive drug to avoid confounding results.
After surgical draping in the operating room, the prepuce was aseptically retracted to expose the glans as the first step for penoplasty. The prepuce excoriate of the attached subpreputial space between the inner prepuce skin and glans surface was performed like as adhesiolysis. Then the smegma was obtained inside of exposed subpreputial space. Because this smegma was located inside of attached subpreputial space and never had been exposed to outside, we called it as ‘virgin smegma.’
Patients were divided into two groups depending on the presence of smegma in this closed space. Patients were classified as group S (with smegma, n=20) and group C (without smegma, n=20). In group S, two kinds of swab were performed.
A first swab was taken directly from the virgin smegma of the subpreputial space. All the virgin smegma was removed and mixed in normal saline, and the mixture was dispenses in a sterile tube for smegma culture. A second glans swab was taken from the mucosal surface of the foreskin and glans from the inside of the subpreputial space where the virgin smegma had been placed in. In group C, an only glans swab was taken from the mucosal surface of the foreskin and glans inside the subpreputial space.
The swabs were immediately sent to the microbiology laboratory for microscopy, culture, and antimicrobial sensitivity testing. The swabs were inoculated aseptically onto blood agar using a platinum wire loop. All incubations were at 37℃ for 24 hours for aerobic culture and 48 hours for anaerobic culture. Bacteria were isolated, identified, and confirmed by standard bacteriological techniques, and antimicrobial sensitivity pattern was determined using the disc diffusion method [14].
After surgery the patient had routine follow-up schedule for 6 months.
Among 40 boys with concealed penis, 17 had mild to moderate glandular hypospadias. The 40 boys ranged in age from 3 months to 9 years 11 months (mean age, 30.4±26.4 months). By dividing into two groups, the age of group S ranged from 3 months to 6 years 6 months (mean age, 25.0±19.7 months). The age of group C ranged from 8 months to 9 years 11 months (mean age, 35.8±30.8 months) (p=0.048). The virgin smegma is more frequently found in young age. Other than age, the groups had no significantly different characteristics (Table 1).
Among 20 boys, six (30.0%) had a single organism isolated, nine had mixed growth (45.0%), and one had a nonuropathogen growth (5.0%), with no bacteria isolated in four boys (20.0%). Thirty-one bacterial uropathogens were isolates from 15 boys, comprising 16 gram-negative isolates (51.6%) and 15 gram-positive isolates (48.4%). The most commonly isolated gram-negative uropathogen was E. coli (8, 25.8%), and the commonly isolated gram-positive uropathogen bacteria were Enterococcus faecalis (6, 19.4%) and Enterococcus avium (4, 12.9%) (Table 2).
Most of the organisms isolated were sensitive to the commonly used antimicrobial agents in clinical practice, except ampicillin in gram-negative isolates, and erythromycin, penicillin-G and tetracycline in gram-positive isolates (Supplementary Table 1). Over half of the organisms isolated (19/31, 61.3%) were multi-drug resistant, and comprised E. coli (n=6 isolates), Enterococcus raffinosus (n=3), E. faecalis (n=2), E. avium (n=2), Staphylococcus epidermis (n=2), Enterobacter cloacae (n=1), Proteus mirabilis (n=1), Morganella morgana (n=1), and Streptococcus mitis/oralis (n=1).
In group S, 12 boys had 22 bacterial isolates in the glans. A single isolate was found in six boys (30.0%). There were 10 gram-positive bacteria and 12 gram-negative bacteria. The commonly isolated uropathogen bacteria were E. coli (6/22, 27.3%), E. avium (5/22, 22.7%) and E. faecalis (4/22, 18.2%).
In group C, 13 boys had 21 bacterial isolates in the glans. Each of the different type of single isolate was found in six boys (30.0%). There were 14 gram-positive bacteria, 6 gram-negative bacteria and 1 fungus. The commonly isolated bacterial uropathogens were E. faecalis (6/21, 28.6%), E. avium (2/21, 9.5%) and E. raffinosus (2/21, 9.5%). E. coli was isolated just one in group C.
When we compared the uropathogen growth in the group S (n=12) and the group C (n=13), there is statistically significant difference in the proportion of patients with E. coli isolated from the glans between the group S (n=6) and the group C (n=1) by Fisher's exact test (the two sided) (p=0.030) (Table 3).
The patients did not have any kind of postoperative complication such as surgical site infection or UTI.
UTI is the most common bacterial infection in children. The factors that contribute to UTI development in children are not yet completely understood. Host features, bacterial characteristics, and immune status all contribute to the development of pediatric UTIs [15]. The main pathogen of the UTI is E. coli [6]. Uropathogenic bacteria, particularly P-fimbriated E. coli, adhere well to the inner mucosal surface of the prepuce [16]. However, studies on strains of P. mirabilis have not found a relationship between the adhesiveness and origin of the organisms and their association with UTI [517].
This risk appears to correlate with a period during the first 6 months of life when there is an increased amount of uropathogenic bacteria colonizing the prepuce. This colonization appears to decrease and resolve by 5 years of age [5]. In general, circumcision has many health benefits that include decreased risk of UTI [18]. Circumcision reduces the rate of UTI development in the first 6 months of life by almost ten-fold [19]. Therefore, periurethral colonization of uncircumcised child could be an important first step for an ascending UTI.
The prepuces fuse with the epithelium of the glans when they are formed in intrauterine life. At birth, their separation is incomplete and continues through childhood. The prepuce is retractable only in 4% of boys at birth and the external urethral meatuses are visible in 50% of newborn babies. Complete preputial retraction occurs only in 20% of infants by 6 months and the prepuces are retracted completely in most boys by 17 years of age [20]. The easier retraction of the prepuce that occurs with increasing age may improve foreskin hygiene and decrease colonization of periurethral aerobic flora.
The concentrations of periurethral aerobic organism decrease with increasing age [21]. The rate of bag positive culture and the infection rate in males decreases 6 to 9 months after birth. There are no general differences in the incidence of infection between circumcised and uncircumcised boys after 12 months of age. In other words, the presence of the prepuce is a highly significant risk factor for UTI in infants up to 12 months of age [46]. This is true regardless of race and socioeconomic circumstance [6].
At first, smegma was thought to be produced by ectopic subpreputial sebaceous glands near the frenulum called Tyson's glands, but these glands were not found [10]. Actually, smegma is a subpreputial collection of desquamated epithelial debris, mixed with mucin, and secretions with a composition including fat (about 27%) and protein (about 13%) [10]. The functions of smegma are moisture and lubricating the subpreputial space [9]. It may contain anti-bacterial enzymes including lysozyme and hormones like androsterone [892223], although this is equivocal [24]. Especially lysozyme, which probably originates from the prostate and seminal vesicles [22], destroy bacterial cell walls and inhibit and destroy candidal species [25]. It also may contain immunologically active chemical compounds such as cathepsin B, lysozyme, chymotrypsin, neutrophil elastase, [26], cytokines [927].
However, the role of smegma in pediatric UTI is not yet completely understood. In a study from Nigeria [28], they found bacterial isolates in smegma swabs from 52 boys ranging in age range from 7 days to 11 years. A single isolate was found in 34 boys (65.4%), eight had a mixed isolate (15.4%), while no bacteria was isolated in 10 boys (19.2%). The commonly isolated gram-positive bacteria were Staphylococcus epidermidis (44.8%) and S. aureus (41.4%) and the most commonly isolated gram-negative bacterium was E. coli (90.5%). Most of the bacterial isolates were multi-drug resistant. They suggest the differences in the organisms from other studies means a local variation due to differences in climate and diet, but also the socio-economic differences in the various populations.
In a study from Turkey [29], smegma swabs from 100 prepubertal healthy boys ranging in age from 2 months to 9 years were collected. The 72 isolates consisted of 54 gram-positive bacteria (75.0%), 17 gram negative bacteria (23.6%), and one (1.4%) Candida isolate. The most commonly isolated gram-negative bacterium was E. coli (41.2%) and the commonly isolated gram-positive bacterium was Enterococcus sp. (57.4%). Most of the bacterial isolates were drug-sensitive.
In this study, most of the organisms isolated were sensitive to common antimicrobial agents in clinical practice, except ampicillin for gram-negative isolates and erythromycin, penicillin-G, and tetracycline for gram-positive isolates. Over half (61.3%) of the organisms isolated were multi-drug resistant.
Recently these multiple-drug-resistant organisms are a major concern about medical and public health issues. Little information about resistance to third-generation cephalosporin as well as multidrug resistance in E. coli is known [30]. Therefore, high detecting rate of multi-drug resistant in smegma is seems to be interesting in this study.
Mycobacterium smegmatis was originally isolated in smegma. The organism is found in dirt and its original isolation from smegma was most probably coincidental [9].
Smegma is easily found when the penile surgery is performed. Exposed smegma could shows similar with preputial skin microbiology. However, in this study we only focus to ‘virgin smegma’ that never has been exposed to the outside. Because the fusion between the prepuces and the epithelium of the glans is a little tight, we thought ‘virgin smegma’ should be sterile. However, this ‘virgin smegma’ already had many kinds of bacterial isolates in our study. Even the rate of E. coli differed according to the presence of smegma. Our study shows in the first time that smegma has bacterial isolates even though it is ‘virgin smegma.’
Despite of the discovery of a variety of organisms from the subpreputial space of boys, the patients don't have UTI symptom or postoperative UTI complication. That points to the fact that colonization does not always lead to infection.
This study has some limitation. The patient age range is quite diverse. This may be due to younger patients having trapped smegma compared to older patients. Moreover, all isolates were some or few growth. Therefore, we are unable to conclude that the bacteria isolated cause UTIs. However, we found that E. coli was more common in the smegma and the subpreputial space of glans with smegma in this study.
References
1. Ginsburg CM, McCracken GH Jr. Urinary tract infections in young infants. Pediatrics. 1982; 69:409–412. PMID: 7070887.

2. Wiswell TE, Smith FR, Bass JW. Decreased incidence of urinary tract infections in circumcised male infants. Pediatrics. 1985; 75:901–903. PMID: 3991278.

3. Wiswell TE, Hachey WE. Urinary tract infections and the uncircumcised state: an update. Clin Pediatr (Phila). 1993; 32:130–134. PMID: 8453827.

4. Wiswell TE, John K. Lattimer lecture. Prepuce presence portends prevalence of potentially perilous periurethral pathogens. J Urol. 1992; 148:739–742. PMID: 1386390.
5. Glennon J, Ryan PJ, Keane CT, Rees JP. Circumcision and periurethral carriage of Proteus mirabilis in boys. Arch Dis Child. 1988; 63:556–557. PMID: 3291784.

6. Herzog LW. Urinary tract infections and circumcision. A case-control study. Am J Dis Child. 1989; 143:348–350. PMID: 2624614.
7. Fussell EN, Kaack MB, Cherry R, Roberts JA. Adherence of bacteria to human foreskins. J Urol. 1988; 140:997–1001. PMID: 2902235.

8. Fleiss PM, Hodges FM, Van Howe RS. Immunological functions of the human prepuce. Sex Transm Infect. 1998; 74:364–367. PMID: 10195034.

9. Van Howe RS, Hodges FM. The carcinogenicity of smegma: debunking a myth. J Eur Acad Dermatol Venereol. 2006; 20:1046–1054. PMID: 16987256.

10. Parkash S, Jeyakumar S, Subramanyan K, Chaudhuri S. Human subpreputial collection: its nature and formation. J Urol. 1973; 110:211–212. PMID: 4722614.
12. Sonnex C, Croucher PE, Dockerty WG. Balanoposthitis associated with the presence of subpreputial “smegma stones”. Genitourin Med. 1997; 73:567. PMID: 9582487.

13. Kayaba H, Tamura H, Kitajima S, Fujiwara Y, Kato T, Kato T. Analysis of shape and retractability of the prepuce in 603 Japanese boys. J Urol. 1996; 156:1813–1815. PMID: 8863623.

14. Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J ClinPathol. 1966; 45:493–496.

15. Cooper CS, Storm DW. Infection and inflammation of the pediatric genitourinary tract. In : Wein AJ, Kavoussi LR, Partin AW, Peters CA, editors. Campbell-Walsh urology. 11th ed. Philadelphia: Elsevier;2016. p. 2926–2948.
16. Wiswell TE. The prepuce, urinary tract infections, and the consequences. Pediatrics. 2000; 105:860–862. PMID: 10742334.

17. Adegbola RA, Old DC, Senior BW. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J Med Microbiol. 1983; 16:427–431. PMID: 6139485.

18. Verit A, Zeyrek FY, Mordeniz C, Ciftci H, Savas M. Status of high-risk oncogenic human papillomavirus subtypes harbored in the prepuce of prepubertal boys. Urology. 2012; 80:423–426. PMID: 22554592.

19. Schoen EJ, Colby CJ, Ray GT. Newborn circumcision decreases incidence and costs of urinary tract infections during the first year of life. Pediatrics. 2000; 105:789–793. PMID: 10742321.

20. Oster J. [The prepuce in Danish schoolboys. Incidence of preputial adhesion, phimosis, and smegma]. Nord Med. 1968; 80:1318–1322. Danish. PMID: 5688182.
21. Bollgren I, Winberg J. The periurethral aerobic bacterial flora in healthy boys and girls. Acta Paediatr Scand. 1976; 65:74–80. PMID: 766562.

22. Prakash S, Rao R, Venkatesan K, Ramakrishnan S. Sub-preputial wetness--its nature. Ann Natl Med Sci (India). 1982; 18:109–112.
24. Waskett JH, Morris BJ. Re: 'RS Van Howe, FM Hodges. The carcinogenicity of smegma: debunking a myth An example of myth and mythchief making. J Eur Acad Dermatol Venereol. 2008; 22:131. author reply 131-2. PMID: 18182000.

25. Tobgi RS, Samaranayake LP, MacFarlane TW. In vitro susceptibility of Candida species to lysozyme. Oral Microbiol Immunol. 1988; 3:35–39. PMID: 3268748.

26. Fröhlich E, Schaumburg-Lever G, Klessen C. Immunelectron microscopic localization of cathepsin B in human exocrine glands. J Cutan Pathol. 1993; 20:54–60. PMID: 8468418.

27. Ahmed AA, Nordlind K, Schultzberg M, Lidén S. Immunohistochemical localization of IL-1 alpha-, IL-1 beta-, IL-6- and TNF-alpha-like immunoreactivities in human apocrine glands. Arch Dermatol Res. 1995; 287:764–766. PMID: 8554390.

28. Anyanwu LJ, Kashibu E, Edwin CP, Mohammad AM. Microbiology of smegma in boys in Kano, Nigeria. J Surg Res. 2012; 173:21–25. PMID: 21872267.

29. Balci M, Tuncel A, Baran I, Guzel O, Keten T, Aksu N, et al. High-risk oncogenic human Papilloma virus infection of the foreskin and microbiology of smegma in prepubertal boys. Urology. 2015; 86:368–372. PMID: 26199167.

30. Ahoyo AT, Baba-Moussa L, Anago AE, Avogbe P, Missihoun TD, Loko F, et al. [Incidence of infections dues to Escherichia coli strains producing extended spectrum betalactamase, in the Zou/Collines Hospital Centre (CHDZ/C) in Benin]. Med Mal Infect. 2007; 37:746–752. French. PMID: 17434702.
SUPPLEMENTARY MATERIAL
Scan this QR code to see the supplementary material, or visit https://www.icurology.org/src/sm/icurology-60-127-s001.pdf.

Table 1
Patient's characteristics of two groups
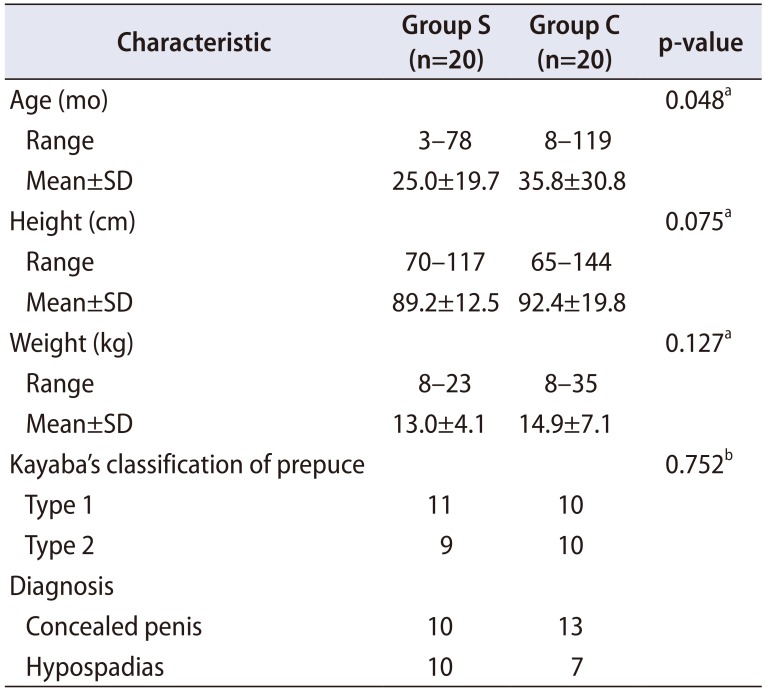
Table 2
Uropathogens isolated from smegma




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download